Deciphering the role of adipose tissue in breast cancer progression and regulation by obesity
Principal investigators
Frédérique Fallone, Charlotte Vaysse and Camille Attané
Other staff involved
Stéphanie Dauvillier (Engineer), Marie Rebeaud (PhD student, Fellow of the Ligue contre le Cancer), Caroline Bouche (Breast Cancer surgeon, PhD Student)
Collaborators
- Dr Anne Bouloumié’s group, Institute of Metabolic and Cardiovascular diseases (I2MC), Toulouse
- Pr Philippe Valet’s group, RESTORE Institute, Toulouse
- Dr Camille Franchet, Pathology Department of the University Toulouse Cancer center (headed by Pr P. Brousset)
- Pr Olivier Piot’group, Translational BioSpectroscopy, Reims
- Dr Daniela Quail, Mac Gill University, Montreal, Canada
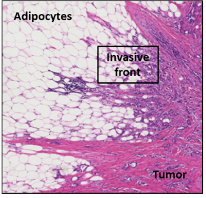
Figure 1 – Adipocytes are major components of the mammary tumoral microenvironment. Hematoxylin/eosin staining of the invasive front in breast cancer. When cancer become invasive, tumor cells (pink/mauve) come into contact with the adipose tissue (white cells).
Breast cancer is the most common cancer in women worldwide. Women with breast cancer who are overweight or obese have a shorter disease-free survival, a higher mortality and a decreased response to chemotherapy, relative to their lean counterparts.
Mammary adipose tissue is a major component of the breast tumor microenvironment. Our team has been among the first to characterize the modifications of tumor surrounding adipocytes. We named them CAAs for Cancer-Associated adipocytes, a term that is now widely used by the international community (Figure 1). CAAs exhibit a decrease in size and lipid content and an activated phenotype marked by an increase expression of pro-inflammatory cytokines and proteins involved in the extra-cellular matrix remodeling (Dirat et al. 2011 Cancer Res, 500 citations in Web of Science, Top 1%). Upon prolonged exposure to cancer cells, adipocytes will dedifferentiate into fibroblasts and as such represents a sub-population of the Cancer-Associated Fibroblasts (CAFs) (Bochet et al. 2013 Cancer Res, Top 1 % Web of Science). Initially identified by our team in breast cancer, it is now widely acknowledged, in all solid tumors, that the invasion of proximal adipose tissue leads to profound delipidation of adipocytes, that could ultimately lead to the accumulation of fibroblast-like cells. In turns, CAAs promote cancer aggressiveness (especially the invasive capacities of cancer cells) and resistance to conventional chemotherapy through their ability to secrete different soluble factors including interleukin 6 (Dirat et al. 2011 Cancer Res; Bochet et al. 2013 Cancer Res; Lehuédé et al. 2019 Breast Cancer Res, 2019 ; for review, Duong et al. 2017 Oncotarget).
One of the most specific and emerging mechanism regarding the role of mature adipocytes in the tumor microenvironment involves the ability of cancer cells to advantageously exploit the nourishing role of adipocytes. In breast cancer, we have demonstrated that tumor cells induce lipolysis in adipocytes leading to the release free fatty acid (FA) that are taken up by tumor cells. These FA trigger a complex metabolic remodeling in cancer cells (Figure 2) increasing their invasive and metastatic abilities (Wang et al. JCI Insight, Top 1% Web of Science). Again, this metabolic crosstalk has now been demonstrated in a wide range of models such as ovarian, colon and prostate cancers as well as melanoma, and we have recently highlighted the importance of this concept in a review (Attané and Muller. 2020 Trends Cancer).
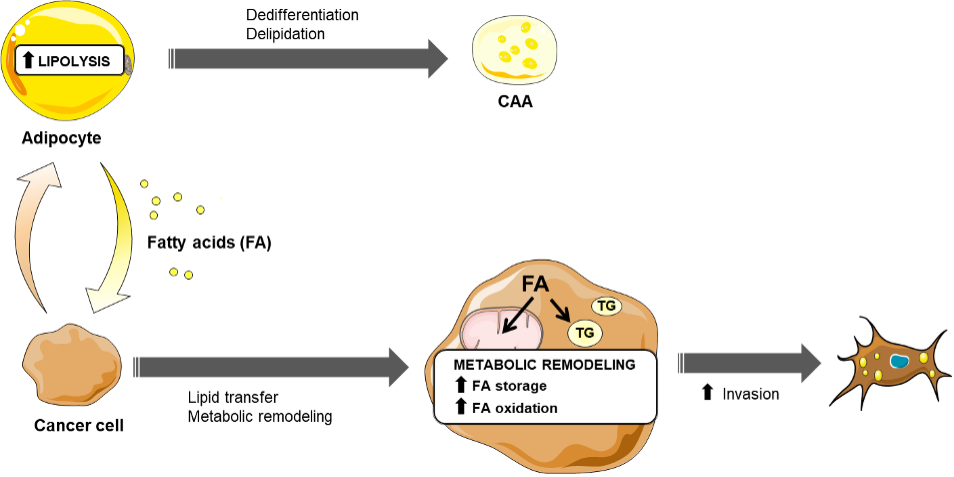
Figure 2. Tumor progression is promoted by tumor cell uptake of lipid released by adipocytes at the invasive front. Schematic representation of the metabolic symbiosis that occurs at the invasive front. Tumor cells release lipolytic signals to transform adipocytes into CAAs. In turns, adipocytes release Fatty Acid (FA). The lipids are internalized and stored as triglycerides (TG) in tumor cells and when needed the tumor cells release FA that are used for fatty acid oxidation (FAO). FAO contributes to increase the invasive abilities of breast cancer cells. Adapted from Attané and Muller. 2020 Trends Cancer.
Our current hypothesis is that this metabolic symbiosis plays a key role in the tumor-promoting effect of adipocytes. We have recently set up a 3D coculture system of mammary adipocytes obtained from lean and obese patients. This model is used: i) to study the metabolic crosstalk with triple negative breast tumor that appears to exhibit metabolic heterogeneity and ii) to study if this metabolic crosstalk is regulated by obesity.
Selected publications
- Dirat B et al. (2011) Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res (Top 1% Web of Science)
- Bochet L et al. (2011) Cancer-associated adipocytes promote breast tumor radioresistance. Biochem Biophys Res Commun
- Bochet L et al. (2013) Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res (Top 1% Web of Science)
- Vaysse C et al. (2017) Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer
- Wang YY et al. (2017) Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight (Top 1% Web of Science)
- Fallone F et al. (2018) Breast cancer, obesity and adipose tissue: a high-risk combination. Med Sci (Paris)
- Lehuédé C et al. (2019) Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP). Breast Cancer Res

