Deciphering the role of bone-marrow adipocytes in bone metastasis
Principal investigator
Camille Attané
Other staff involved
Sauyeun Shin (post-doc, fellow of the National Cancer Institute), Marine Hernandez (PhD Student in Oncology), Mohammed Moutahir (Technician, CNRS)
Collaborators
- Nicolas Reina (Orthopedic surgery unit, Toulouse Hospital)
- Jean-Charles Portais and Justine Bertrand-Michel (Toulouse Metabolomic and lipidomic Platform)
- Marc Poirot’s team (CRCT, Toulouse)
- Philippe Valet and Cédric Dray (Restore Institute, Toulouse)
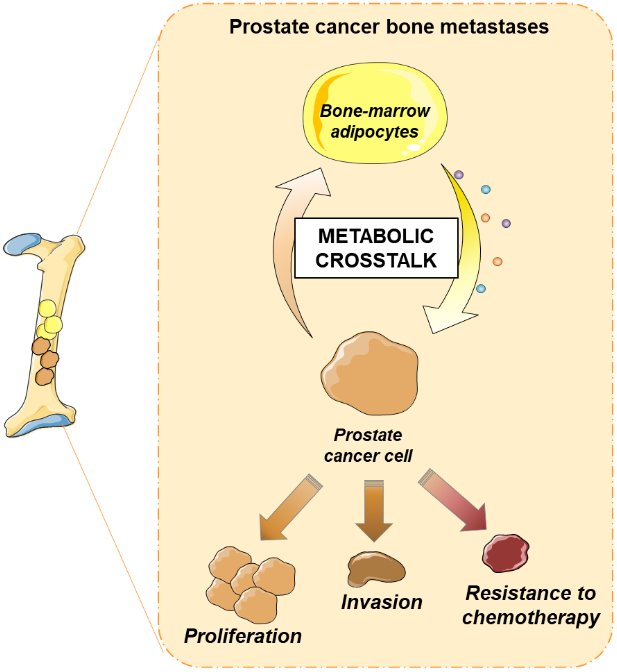
Bone is a frequent site of metastases and many existing therapies are ineffective at metastatic bone sites. Accumulating evidences suggest that bone marrow (BM) microenvironment act as a regulator of cancer cell homing to bone and bone metastasis outgrowth. Inhibition of cancer cells/microenvironment crosstalk represent underexplored potential treatment for bone metastasis.
Adipocytes (BM-Ads) are a major component of the BM microenvironment since they occupy 70% of the total volume of adult BM. These cells are unique in the BM microenvironment due to their secretory and metabolic specificities.
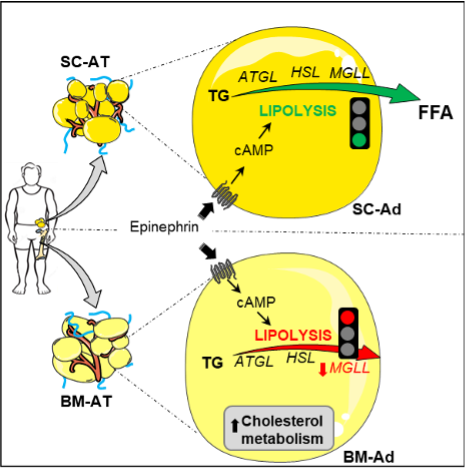
While BM-Ads morphologically resemble classical SC-Ads, they display a very specific lipid metabolism characterized by a defect in lipolytic activity and a cholesterol-oriented metabolism. Attané et al. 2020 Cell Rep
Until now, studying the crosstalk between BM-Ads and tumor cells was clearly hampered by the limited data concerning BM-Ad phenotype in humans and the lack of adequate models for in vitro studies. Thus, we performed the first metabolic characterization of human BM-Ads using a combination of proteomic and lipidomic approaches in collaboration with orthopedic surgeons of Toulouse’s University Hospital (Attané et al. 2020 Cell Rep). We found that BM-Ads morphologically resemble paired subcutaneous adipocytes (SC-Ads; used as a control) but display distinct lipid metabolic features. Our results showed that BM-Ads i) are devoid of lipolytic function, due to a profound down-regulation of MGLL (monoacylglycerol lipase), a lipase involved in the last step of the lipolysis pathway and ii) exhibit a cholesterol-orientated metabolism due to an enrichment in proteins involved in cholesterol synthesis, transport and hydrolysis as well as metabolization into oxysterols. Therefore, our results explain for the first time, at cellular and molecular levels, why BM-Ads behave like a preserved lipid source under caloric restriction.
We have also demonstrated recently that BM-Ads might favor the homing of CCR3 expressing prostate cancer (PCa) cells through their ability to secrete the CCL7 chemokine (Guerard et al. 2021 Int J Mol Sci). Interesting the CCR3/CCL7 axis is also involved in local PCa invasion in a process dependent of the secretion from mature adipocytes of the periprostatic adipose tissue (Laurent et al. 2016 Nat Commun). The implication of CCR3 in both local and distant invasion of PCa highlights the interest of use of CCR3 inhibitors in PCa.
Our current project aims to decipher the role and nature of the metabolic cross talk between PCa cells and BM-Ads in bone metastasis outgrowth in order to propose innovative drug targets for the treatment of PCa bone metastases. We recently reviewed how tumor cells affect the in vitro and in vivo phenotype of BMAds and the signals emanating from BMAds that are susceptible to modulate tumor behavior with a specific emphasis on their metabolic crosstalk with cancer cells (Hernandez et al. 2022 Cancer Metastasis Rev). This project has been funded in 2020 by the French National Cancer Institute and is supported by the Ligue Nationale contre le Cancer (labeled team 2020-2025).
Selected publications
- Hernandez M et al. (2022) The role of bone marrow adipocytes in cancer progression: the impact of obesity. Cancer Metastasis Rev (invited review)
- Attané C*, Estève D* et al. (2021) A protocol for human bone marrow adipocyte isolation and purification. STAR Protoc
- Attané C et al. (2020) Human Bone Marrow Is Comprised of Adipocytes with Specific Lipid Metabolism. Cell Rep
- Guérard A et al. (2021) The chemokine receptor CCR3 is potentially involved in the homing of prostate cancer cells to bone : implication of bone -marrow adipocytes. Int J Mol Sci

