Mechanisms of in vivo electro-permeabilization
Electropermeabilization, gene transfer and modulation of gene expression in tissues in vivo
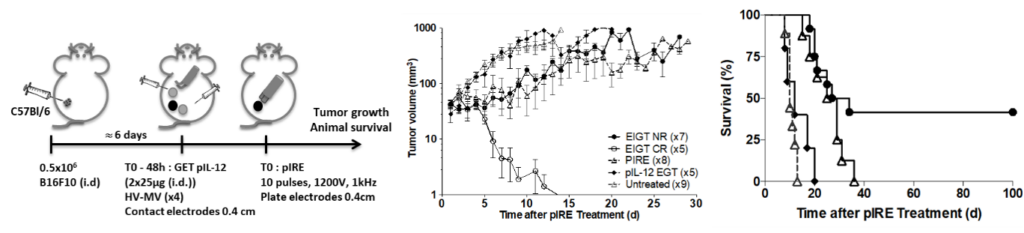
Tissues in living mice such as tumors, muscles and skin are more complex than cells or spheroids. We have shown in vivo an efficient electrotransfer of different types of molecules (DNA, shRNA, siRNA, cis-platinum, bleomycin…) by non-invasive optical imaging. We have also shown that gene expression could be modulated in vivo in muscle by different types of molecules (shRNA, siRNA). These studies have been also performed in sub-cutaneous tumors (mouse melanoma and fibrosarcoma). The results show that the EP parameters leading to efficient delivery vary from tissue to tissue.
In the context of gene electrotransfer (GET), we have studied the effect of new electric parameters on the expression, bio-distribution and safety of different transgenes in mouse skin by EP.
Tissues in living mice such as tumors, muscles and skin are of interest for therapeutic purposes in cutaneous cancers (melanoma and sarcoids) but also for DNA vaccination. The bio-distribution, the stability and the route of administration of these molecules are crucial aspects that must be determined for clinical applications as well as inflammatory response induced by electric field and the implication of the immune system.
Investigators: Project leader, M. Golzio
Collaborations: M. Cemazar (Institute of Oncology, Ljubljana)
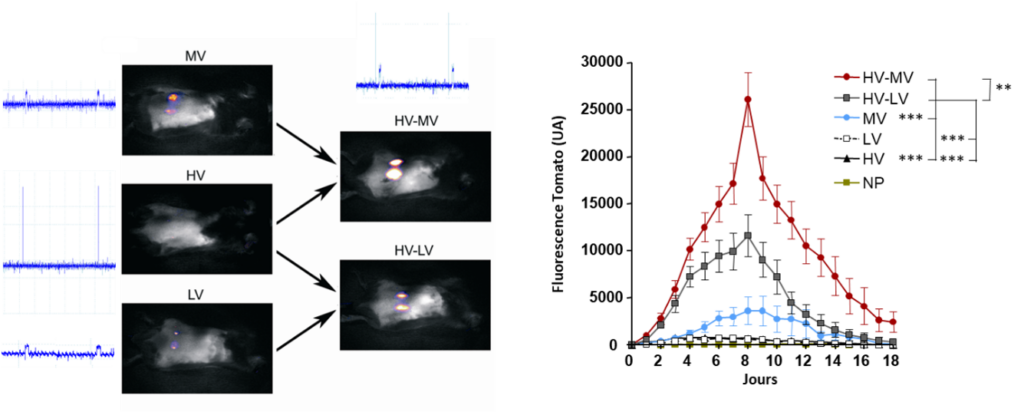
Follow-up of gene transfer of a plasmid encoding the dtomato by fluorescence macroscopy in the mouse skin (Pasquet et al. 2018 Sci Rep)
Physiological effects of electropermeabilization on the vascular network
In vivo EP has also blood flow modifying effect. A decreased blood flow (vascular lock) was observed in muscles and tumors. Our aim is to evaluate the effects of EP on subcutaneous blood vessels morphology (diameter) and dynamics (vasomotricity, permeability and recovery). These features are measured using fluorescently labeled dextran via dorsal skin fold window chamber, intravital fluorescence microscopy imaging and specific image analysis.
Further experiments will be done to characterize the life-time of these processes in normal but also tumor vessels. In order to study these processes, we developed and used fluorescence macro-scopes combined or not with intravital microscopy (conventional or multiphoton microscopy). Thus, we can follow cellular or molecular events on the living animals (from minutes, to months).
Investigators: Engineer, E. Bellard; Project leader: M. Golzio
Collaborations: M. Cemazar (Institute of Oncology, Ljubljana)
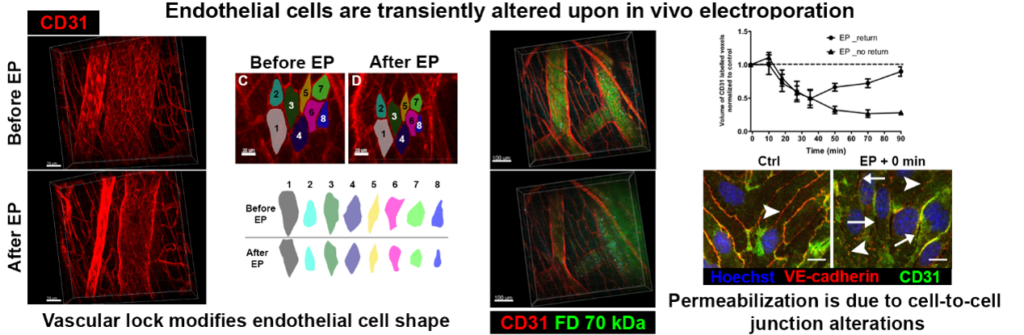
Vascular lock is induced by electroporation in normal vessels. We also observed that the permeability of the blood vessels is affected by the electric field (Markelc et al. 2018 JCR)
DNA electro-vaccination, immune responses and mobilization of dendritic cells
The skin and dermis in particular contain a large number of immune cells to protect the body and induce an appropriate immune response. A new generation of vaccines is associated with the use of “naked DNA”. In vivo electroporation appears to be a way of choice to potentiate the transfer of naked DNA, and thus the resulting vaccination but electroporation can play an important role by itself as an adjuvant.
Further experiments are in progress to characterize the implication of the immune cells in electroporation-based protocols and optimize, thus, the activation of the immune system induced by electropermeabilization itself as well as the stimulation of the immune system induced by electrogenotherapy expressing plasmid coding IL-12 cytokine for example.
Impact of EP and ECT on DC mobilization and activation in the treated tumor is also under investigation. In order to study these processes, cytometry and fluorescence microscopies (conventional or multiphoton microscopy) will be used.
Investigators: Engineer, E. Bellard; Project leader: M. Golzio
Collaborations: M. Cemazar (Institute of Oncology, Ljubljana); S. Guerder (Infinity, Toulouse).