Mechanisms of Membrane Electro-Permeabilization
Our objectives are to give the full comprehension of molecule transfer through membranes taking into account the complexity and characteristics of the cellular microenvironment, and by doing so to develop new approaches and guidelines for the safe and efficient delivery of therapeutic molecules into cells and tissues. Our strategy is to adapt and develop different complementary systems with increasing complexities (model membranes, cells in 2 and 3D culture, multi-cellular spheroids, human reconstructed skin and tissues in living mice) and to integrate various imaging tools to visualize and define the mechanisms involved in the delivery processes of therapeutic or prospectively therapeutic compounds.
Molecular Mechanisms
GUVs (Giant Unilamellar Vesicles) represent a convenient way to study membrane properties such as lipid bilayer composition, surface charge and membrane tension. They offer the possibility to study and visualize membrane processes due to their cell like size in absence of any constraint due to cell cytoskeleton. We already validated the GUVs model showing that different membrane perturbations (pores, vesicles and tubules) are associated to membrane electropermeabilization (Portet et al. 2009 Biophysical J). We directly determined using the GUVs as the basic model of lipid vesicles, the transmembrane potential needed to induce permeabilization, and showed that it is strongly dependent on the lipid composition (Mauroy et al. 2015 Langmuir).
The open questions we now address are: 1) to understand the effect of membrane composition and domains, 2) to determine the uptake process of molecules according to their size and charge and 3) to explore the interaction of electromagnetic fields (Pillet et al. 2018 RSC Advance) as well as ns pulsed electric field with membranes.
Investigators: Technician, L. Hellaudais; Post-Doc, F. Pillet; Project leader: MP Rols
Collaborations: D. Dean (Bordeaux), R. Vézinet (CEA, Gramat), P. Levêque (Xlim, Limoges)
Membrane Organization and Dynamics
Cells in culture have revealed more complex ways involved in molecule electro-mediated uptake than GUVs can do with the puzzling notion of competent micro-domains for molecule uptake. We indeed observed years ago alteration in membrane organization and dynamics, in particular flip-flop of lipids, which may be involved in molecule uptake (Escoffre et al. 2014 Biochim Biophys Acta). A key question about therapeutic molecules concerns their subsequent distribution into the cells. This is of high importance in the case of DNA since expression requires traffic, i.e. translocation of DNA across the membrane, migration through the cytoplasm and finally passage through the nuclear envelop. We previously showed actin polymerisation at the membrane sites where DNA interacts with (Rosazza et al. 2011 Mol Ther). We visualized the intracellular traffic of single molecule thanks to a collaboration with a group leader in that field (A. Zumbusch). We attested the evidence for endocytosis and endosomal trafficking of DNA, showing for the first time, the different routes and complexity of the phenomenon of molecules uptake by pulsed electric field according to their size and charge (Rosazza et al. 2016 Mol Ther Nucleic Acids). We showed that gene expression highly depends on electric field parameters (de Caro et al. 2023 Pharmaceutics). We also used microwave biosensor to monitor and characterize single cells subjected to electroporation and obtain an electronic signature of the treatment efficiency (Tamra et al. 2022 IEEE Trans Biomed Eng).
The questions we now address are to improve the efficiency of nucleic acid delivery by the development of new protocols of pulses delivery with the aim to reduce muscle contraction and pain.
Investigators: PhD students, A. Calvel, A. de Caro, C. Rosazza, A. Tamra; Engineer: G. Alberola; Project leader: MP Rols
Collaborations: A. Zumbush (University of Konstanz, Germany), D. Miklavcic (University of Ljubljana, Slovenia), K. Grenier (LAAS, Toulouse), J.-B. Leroy (LEROYBiotech, Saint-Orens de Gameville)
From Cells to Tissues: 3D cell cultures
Multicellular spheroids are 3D models of cells in culture able to mimic the behaviour of cells in a complex 3D organized system like a tissue. They allow studying more accurately, than isolated cells, what is happening in a model tissue environment, taking into account cell/cell interactions, extracellular matrix and accessibility of molecules. We already showed years ago that they can be used as an original and convenient approach to study the mechanism of electropermeabilization in a tissue (Wasungu et al. 2009 Int J Pharmaceutics; Gibot et al. 2013 J Control Release). We observed, as occurring in patients, that normal cells are less sensitive than tumor cells to electrochemotherapy (ECT) i.e. the administration of anticancer drugs (bleomycin or cisplatin) followed by the local delivery of high-voltage electric pulses (Frandsen et al. 2015 PLOS One).
In the project NUMEP, composed of computer scientists (Inria MONC) and radiologist (CHU J. Verdier), we provided new insights in understanding biologically and in modeling numerically the effect of electroporation on tumors, in order to provide numerical tools enriched by biological knowledge that help the clinical applications of electroporation in cancer treatment (Collin et al. 2022 AIMS bioengineering). It is crucial to determine the differences between healthy cells, proliferative and quiescent cancer cells, as well as the influence of electric pulses on the regrowth of the spheroids. We investigate and quantify the efficiency of ECT and gene electrotransfer on spheroids composed with human tumor cells, healthy cells or both. Our ongoing project MECI granted by Plan Cancer has started in February 2022 for 3 year with the same partners (Inria MONC, Interventional Radiology unit of AP-HP, Avicenne) and the Radiology and nuclear medicine service of the University hospital of Poitiers (Tasu et al. Diagn Interv Imaging. 2022).
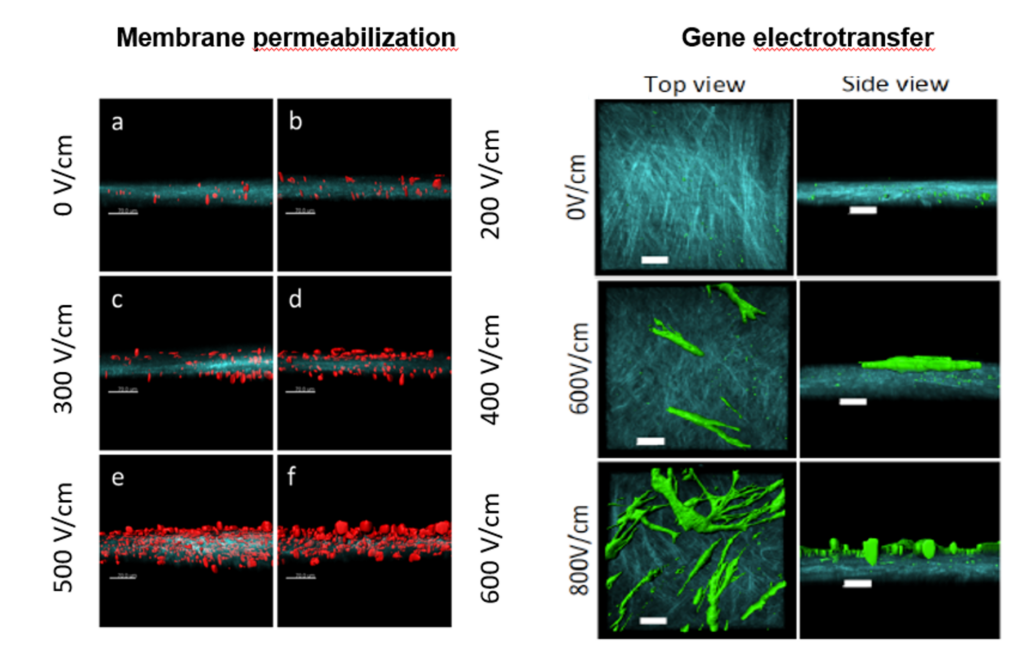
3D dermal tissue electropermeabilization, from Madi et al. 2015 J Membrane Biol (left) and Madi et al. 2016 Cure Gene There (right).
In addition to multicellular spheroids, we also implemented a human skin model and used competitive microscopy techniques (confocal, two-photon and atomic force microscopies) to perform real time observations. We showed relevance of the models to address and improve the electrotransfer processes. We could visualize the extracellular matrix and define electric field conditions allowing the efficient biodistribution of molecules in both models and optimum cell plasma membrane permeabilization for their uptake (Madi et al. 2014, 2016). We revealed the importance of endogenous extracellular matrix in biomechanical properties of human skin model (Pillet et al. 2017 Biofabrication). In addition to human dermal tissue, we also implemented human epidermis tissue.
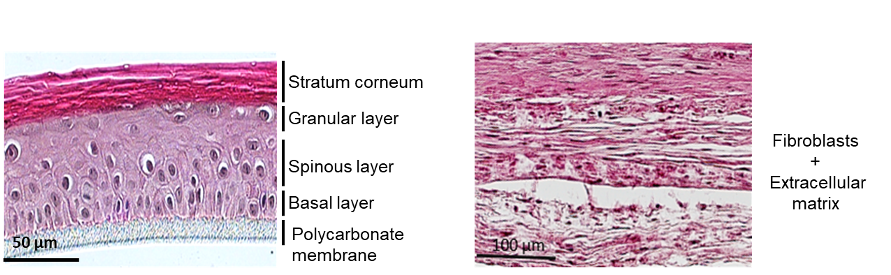
3D epidermal and dermal tissue. Copyright, G. Alberola.
The behavior of melanoma and the role of caveolae along electrochemotherapy protocols is now under investigation in cells and reconstituted skin. Considering the response of caveolae to cell swelling, we hypothesize that the particularities of the caveolae mechanical response and associated parameters in melanoma could explain both the targeted antitumor effect of ECT to melanoma cells and the 20% nonresponse to ECT. This project is developed in collaboration with Dr. C. Lamaze (Curie Institute) as part of the Fondation ARC project.
Investigators: PhD students, A. de Caro, M. Madi; Postdoc: L. Gibot, F. Pillet; Engineer: G. Alberola; Project leaders: MP Rols and J Kolosnjaj-Tabi
Collaborations: J. Gehl (Denmark), D. Dubuc (LAAS, Toulouse), P. Vicendo (IMRCP, Toulouse), C. Lamaze (Institut Curie, Paris), C. Poignard (Inria, Bordeaux), O. Seror (CHU Verdier, Bondy), J.-P. Tasu (CHU Poitiers) – TRI Platform
Bacterial spores and biofilms inactivation by microsecond PEF, electric arcs and nanoparticles
Bacterial spores are one of the most resistant life forms known, extremely resistant to chemical, environmental and physical stresses. This considerable resilience and endurance is explained by a highly dehydrated structure which included genomic material protected and surrounded by bacterial envelope organized in successive multilayers. Bacterial spores can cause respiratory infection, food contamination and fatal paralytic illness.
We combined atomic force microscopy (AFM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to image at the nanoscale, the cell-envelope disorganization of spores. We showed that Pulsed Electric Field to eradicate bacteria, since electric fields can destroy the membrane, affect the cell wall, induce DNA damage (Pillet et al. 2016 Sci Rep; Lamarche et al. 2018 PLOS One) and destabilize the extracellular matrix allowing resistant bacteria to be eradicated. PEF have a direct effect of on protein architecture of the coat. AFM confirmed these results and showed a flattening of ridges on the coat surface. These results open a new avenue for inactivation of bacteria by direct cell-wall targeting effects.
We are also using magnetic silica-coated iron oxide nanochains as photothermal agents to disrupt the extracellular matrix to eradicate cancer cells (Kolosnjaj-Tabi et al. 2019 Cancers). This approach shows great promise to eradicate bacterial biofilms.
The main translational research aspects of our research are therefore developing in collaboration with medical and industrial partners in the Midi-Pyrénées area and among different French and global networks.
Investigators: PhD students, C. Lamarche, M. Bocé; Postdoc: F. Pillet; Engineer: C. Da Silva; Project leaders: J Kolosnjaj-Tabi and MP Rols
Collaborations: G. Demol (ITHPP, Thegra), I. Malfant (LCC, Toulouse), E. Dague (LAAS, Toulouse), D. Miklavcic (Ljubljana, Slovenia)
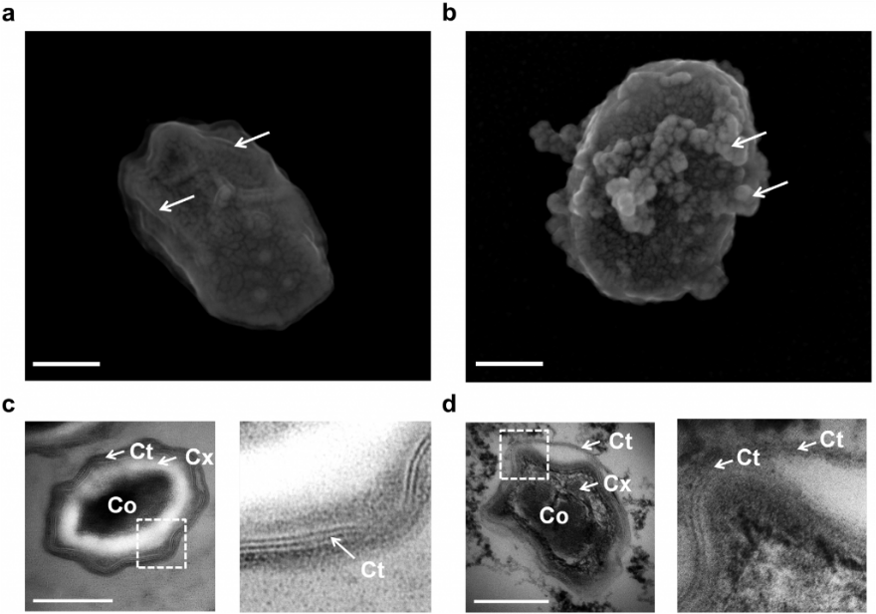
PEF induced internal damage to spores (from Pillet et al. 2016)
Conclusion
We expect to go beyond simple “electroporation” protocols by developing strategies, based on well-defined EP parameters and combined complementary approaches, to “electromanipulate” cells in vivo for instance reversibly or irreversibly permeabilize them (to permit drug delivery or illness eradication) taking into account the cell and tissue microenvironment to address cell specificities, targeting the physical hallmarks of the microenvironment to treat tumors and pathogens. Exposure of cells and tissues to electric field pulses allows modifying the microenvironment and enhancing drug delivery. Defining how direct or indirect effects of electric pulses can selectively and specifically modify ECM, intercellular junctions, membranes, cell migration, and immune cell response will improve therapies. Tissue engineering approaches are used to study and improve strategies for drug delivery by physical methods such as pulsed electric fields, radiofrequencies, magnetic nanoparticles and plasma.

