Molecule Delivery, Targeting and Diagnostic: new tools
Nanotheranostic tool for imaging and treatment of epithelial cancers
We are developing a nanocontainer protein as a vectorization system for the targeted delivery of therapeutic molecules and as a diagnostic imaging tool. The molecular marker specifically detected by this protein is characteristic of epithelial cancers. In collaboration with Toulouse Oncopole surgeons, we have chosen to provide proof of concept of this nano-tool efficacy on human ovarian adenocarcinoma. We validated the biochemical characterization of this nanocontainer. Four chemotherapeutic drugs were successfully confined inside the protein cavity and successfully delivered to adenocarcinoma cells in vitro. The use of this nanocontainer, as an imaging probe, after its labelling with a near infrared dye (Alexa 647), has been explored in vivo on a mouse model. We highlighted the possibility to detect submillimeter peritoneal xenografts of human tumor. The nanotheranostic approach is currently being developed with IUCT surgeons. This work was initiated as a maturation project with the SATT-ToulouseTechTransfer (2013-2015) and patented. The valorization of this work through the creation of a start-up company with IUCT surgeons is currently being studied (Coustets et al. 2020 Biomaterials).
Investigators: Engineer & technician: M. Coustets, C. Ladurantie; Project leader: L. Paquereau
Collaboration: G. Ferron (IUCT, Toulouse)
Needle-free transdermal delivery
There are various alternative routes (electrical, mechanical, thermal) for transdermal delivery to avoid the use of the injection syringe, which could improve the quality of life of patients with diseases such as diabetes. These methods include, for example, micro-needles, electro-permeabilization, and iontophoresis. Electropermeabilization allows, via the application of an electric field, to temporarily increase the permeability of the skin and consequently to allow the transdermal passage of molecules of high molecular weight.
The goal of this ARN project CARBO2DERM is to design and realize a nanocomposite patch based on carbon nanotubes (CNT) to store, but also releasing a drug when it is subjected to electrostimulation. To do this, different polymers and different shaping techniques have been explored and developed to demonstrate the feasibility of our approach.
Investigators: Engineer, E. Bellard; Project leader: M. Golzio
Collaborations: E Flahaut (CIRIMAT, Toulouse); Z Valdez (LAPLACE, Toulouse)
Optical imaging: multispectral, far-red fluorescent dyes, bioluminescence, intravital imaging and Speckle dynamic systems
Optical imaging of small animals allows observation of kinetic fluorescent events in time and space on the scale of a whole body, an organ or a cell. It enables detection and quantification to monitor gene expression, gene regulation, tumor growth and metastasis on a scale of days or weeks. We use and develop different approaches (multispectral imaging, fluorescent dyes emitting in the far red, bioluminescence).
The properties of the light can also be used. In collaboration with the ONERA, we develop a Tumor Microvasculoscope (TMV) based on Dynamic Polarised Speckle Acquisition (DYPSA). This process has been patented ( Brevet 01/02/2017 n° FR 17 50833).
To overcome limitations induced by the skin, we also adopted the use of intravital microscopy with the implantation of a window chamber or dorsal skinfold chamber on the back of the mice. It allows the monitoring of fluorescent cell events in the skin or a cutaneous implanted tumor on several days in the same animal. Other window models exist that enable cells monitoring in deeper orthotopic tumors like abdominal window that we want to implement for in vivo electropermeabiliszation applications.
Investigators: Engineer, E. Bellard; Project leader: M. Golzio
Collaborations: X. Orlik (ONERA, Toulouse)
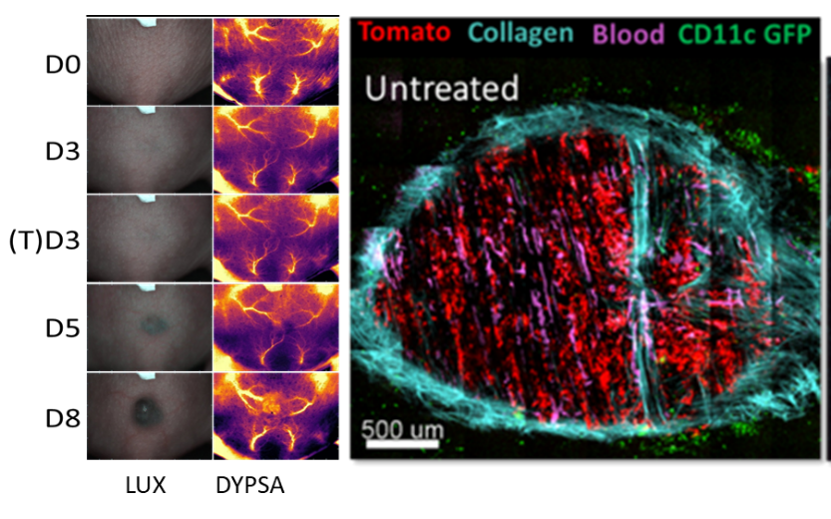
Tumor development pictures acquired with TMV on the same mice. First line correspond at phase contrast picture (LUX) and second line at dynamic polarized speckle acquisition (DYPSA). Tumor in dorsal windows chamber (DWC) observed with 2-photon microscopy (unpublished data).
Nanoparticle-based physical treatments to dismantle the extracellular matrix and improve cancer chemotherapy
Cancer is a devastating disease, and current therapeutic approaches can be inefficient. Poor therapeutic outcomes are not only due to lack of efficiency of therapeutic agents towards cancer cells, but also due to the impenetrability of the tumor mass to conventional drugs. Such impermeability of the tumor stroma is due to the dense extracellular matrix, particularly the collagen network, which prevents the penetration of medicines and immune cells into the tumor.
The primary role of the extracellular matrix is to provide structural and biochemical support to surrounding cells. In addition, studies indicate that the matrix generates mechanical forces, which contribute to cancer progression and resistance to treatment.
In order to dismantle the collagen matrix and alleviate mechanical constraints to improve cancer therapy, this project presents an array of methods based on nanoparticle-mediated physical approaches, which could be used to treat highly desmoplastic tumors.
The objective of this project is to assess the efficacy of spatially and temporally controlled nanoparticle-based treatments exploiting the intrinsic physical properties of nanosized injectable semiconducting (iron oxide) and conducting (gold) nanoparticles. These nanoparticles generate heat and mechanical forces when exposed to physical stimuli, and could disorganize the desmoplasia. Moreover, the same nanoparticles act as “injectable antennas”, which can locally amplify the electric field to potentiate the electroporation of cells and increase the delivery of cytotoxic drugs in a minimally invasive manner to deep-seated tumours, via nanoparticle-enhanced electrochemotherapy. In a multifactorial approach, combining thermal, mechanical and electrical actions, this project aims to dismantle the tumor stroma and sensitize cells to treat cancers. Presented approaches represent a considerable methodological step change and would represent a major breakthrough in the field of electroporation-based therapies and treatments.
Proposed strategies will be optimized and tested in vitro in two- and three-dimensional cellular models, and in vivo in preclinical murine models of highly desmoplastic tumors, with the goal of determining how altered extracellular matrix and, concomitantly, altered tumor mechanics, could impact the outcome of tumor treatment. The efficacy of the treatment will be monitored over time from the ultrastructural level to the whole body level.
Investigators: Project leader, J. Kolosnjaj-Tabi
Collaborations: O. Seror (CHU Verdier, Bondy), J.-P. Tasu (CHU Poitiers), S. Kralj (University of Ljubljana, Slovenia)

