Phagocyte Architecture and Dynamics
Our team combines cutting-edge techniques in optical and electron imaging, material science, cell mechanics and intra-vital imaging to elucidate how phagocytes, in particular macrophages and osteoclasts, interact with the extracellular matrix, to decipher the mechanisms of macrophage migration and phagocytosis and investigate how HIV-1 manipulates phagocyte cell-to-cell spread.
In the long term, our projects will allow the identification of new targets to control the interactions of macrophages with their environment, which will be valuable against both cancer and HIV infection.
We investigate how phagocytes interact with their microenvironment, in physiological and pathological contexts including cancer and infectious diseases.

Using intravital microscopy, our team revealed that macrophages use the mesenchymal mode of migration to infiltrate dense tumors and that inhibitors of matrix metalloproteases both decreased the number of tumor-associated macrophages and tumor growth (Gui et al. 2018 Cancer Immunol Res). Podosomes are cell adhesion structures involved in the degradation of the extracellular matrix and the mesenchymal migration of macrophages. They are composed of a submicron core of actin filaments surrounded by a ring of integrin-based adhesion complexes.
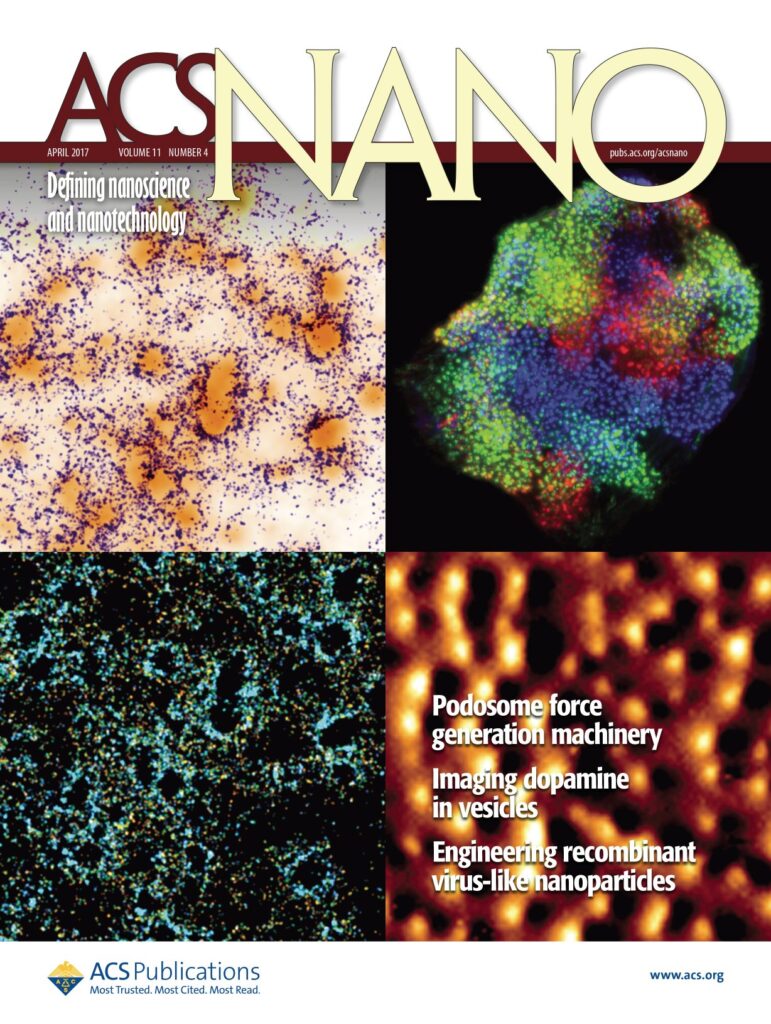
Thanks to a method that we called protrusion force microscopy, we demonstrated that podosomes generate protrusive forces that are proportional to the stiffness of the extracellular matrix (Labernadie et al. 2014 Nat Commun; Proag et al. 2015 ACS Nano; Labernadie et al. 2022 Nat Commun), and involve a balance of forces between core protrusion and a traction at the adhesion ring (Bouissou et al. 2017 ACS Nano). In bone degrading osteoclasts, we could show that there is a local coordination of podosome cores within micrometer-scale islets (Portes et al. 2022 Elife). We also developed a device combining microchannels and pillars and reported that forces are redirected from inwards to outwards with increased cell confinement (Desvignes et al. 2018 Nano Lett). More recently, we could demonstrate, in close collaboration with Marion Jasnin and Serge Dmitrieff, that nano-scale forces by podosomes can be explained by the elastic energy stored in podosome actin networks (Jasnin et al. 2022 Nat Commun).
We recently investigated the function in macrophages of ERM proteins, for Ezrin, Radixin and Moesin, which play a central role in establishing a mechanical link between the plasma membrane and actin filaments. Surprisingly, in the absence of all ERMs, macrophages had no migration defects. Furthermore, unlike all the other cell types studied previously, the loss of ERMs in macrophages does not affect the mechanics of their cortex (Verdys P. et al. EMBO J. 2024). In contrast, moesin (but not the other ERM proteins) plays a negative regulatory role in osteoclast fusion and function (Dufrançais O. et al. J Cell Biol, 2025).
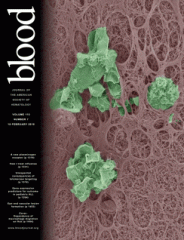
We showed that the HIV-1 protein Nef modulates the migration of macrophages both in vitro and in vivo, and favors virus dissemination by enhancing the mesenchymal migration and by modulating podosome structure and function (Vérollet et al. 2015 Blood). In addition, we observed that osteoclasts are productively infected by HIV-1. The virus strongly alters podosome organization in osteoclasts, leading to enhanced bone resorption activity (Raynaud-Messina et al. 2018 PNAS). These observations likely explain macrophage accumulation in several tissues of HIV-1 infected patients and why they suffer from osteolysis. Macrophages are also the main host cells for Mycobacterium tuberculosis (Mtb). In the context of tuberculosis, we reported that macrophage mesenchymal migration is enhanced, and associated with an accumulation of Mtb-permissive macrophages in lungs (Lastrucci et al. 2015 Cell Res). We also showed that, in tuberculosis microenvironment, the formation of tunneling nanotubes (TNT) by macrophages is increased. When these macrophages are subsequently infected by HIV-1 the virus spread between cells using TNT and the lectin Siglec-1/CD169. These mechanisms could explain how tuberculosis enhances HIV-1 pathogenesis in co-infected patients (Souriant et al. 2019 Cell Rep; Dupont et al. 2020 Elife; Dupont et al. 2022 J Leuk Biol). More recently, we revealed a novel and efficient mechanism of tissue macrophage infection by HIV-1 via the fusion with infected CD4 T lymphocytes (Mascarau et al. 2023 J Cell Biol).
Team members
Research Scientists
Fabrice Dumas (University)
Arnaud Labrousse (University)
Véronique Le Cabec (CNRS)
Renaud Poincloux (CNRS)
Christel Vérollet (Inserm)
Research Engineer
Pierre-Jean Bordignon
Arnaud Métais (CNRS)
Post-doctoral Fellow
Océane Dewingle
Jose Manuel Sanchez Lopez
PhD Students
Dimitra Bozoglou
Clara Deyts
Natacha Faivre
Camille Gorlt
Eleonora Ledaki-Engonopoulou
Marianna Plozza
Myriam Razouk
Merzouk Zidane
Dufrançais O et al. (2025). Moesin controls cell-cell fusion and osteoclast function. J Cell Biol.
Verdys P et al. (2024) Ezrin, radixin and moesin are dispensable for macrophage migration and cortex mechanics. Embo J.
Mascarau M et al. (2023) Productive HIV-1 infection of tissue macrophages by fusion with infected CD4+ T cells. J Cell Biol
Portes M et al. (2022) Nanoscale architecture and coordination of actin cores within the sealing zone of human osteoclasts. Elife
Jasnin M et al. (2022) Elasticity of dense actin networks produces nanonewton protrusive forces. Nat Commun
Dupont M et al. (2020) Tuberculosis-associated IFN-I induces Siglec-1 on tunneling nanotubes and favors HIV-1 spread in macrophages. Elife
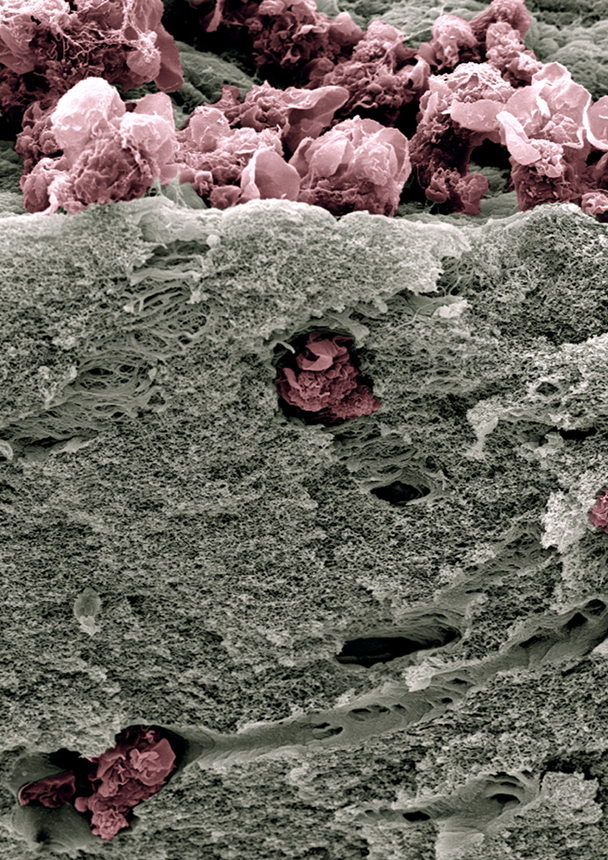
Scanning electron micrography of human monocyte-derived macrophages (pink) that have infiltrated a thick layer of Matrigel® (grey) for 72h. They degrade the extracellular matrix and dig tunnels. © Renaud Poincloux
Prizes
Press Releases
11/2025
Camille Gorlt (PhD) and Maxime Pingret (PhD) were awarded best oral and poster presentation prizes, respectively, at the IPBS PhD 2025 symposium.
05/2025
Marianna Plozza was awarded a Zellidja grant for her PhD work !
05/2025
Camille Gorlt (PhD) was awarded a price for best PhD Talk among 30, at the meeting of the Société Française de Biologie des Tissus Minéralisés, (Reims, France)
05/2024
Marianna Plozza (PhD) and Camille Gorlt (PhD) shared the price for best PhD Talk among 30, at the meeting of the Société Française de Biologie des Tissus Minéralisés, (Bordeaux, France)
06/2023
Sarah Monard (PhD) was awarded a price for best Poster (among over 50) at the last Gordon Research Seminars about Phagocytes (Waterville Valley, USA)
05/2023
Ophélie Dufrançais (PhD) and Marianna Plozza (PhD) shared a price for best PhD Talk among 30, at the meeting of the Société Française de Biologie des Tissus Minéralisés, (Sète, France)
05/2023
Rémi Mascarau (PhD) received one of three “ANRS | Emerging Infectious Diseases / French Society of Virology” thesis prizes for basic and translational HIV research (“Dominique Dormont” prize) that were awarded at the annual Work in Progress (WIP) meeting of AC41 “Host/Virus Interactions” on May 3, 2022 at Paris Santé Campus.
04/2023
Ophélie Dufrançais (PhD) was awarded the Best Abstract Price at the ECTS meeting, Liverpool, UK
Claire Bigot (PhD) was awarded the Best Presentation Price at the EMBO Workshop : ImmunoBiophysics : from fundamental physics to understanding the immune response.
11/2022
Ophélie Dufrancais (PhD) receives the 2022 MINERVA trophies from the F•INICIATIVAS Foundation.
10/2022
Claire Bigot (PhD) and Javier Rey Barroso (Postdoc) received a prize for their oral and poster presentations, respectively, during the 8th Meeting of the Invadosome Consortium (Sètes, France).
04/2022
Ophélie Dufrançais (PhD) was awarded the 2022 Young Researcher Price for her oral presentation during the 22nd French Days of Mineralised tissue biology 2022 (La Baule, France).
02/2022
Rémi Mascarau (PhD) was awarded the 2022 Young Researcher Price of the Treilles Foundation (France).
10/2018
Arnaud Labrousse (Assistant Professor) received the National PEPS Prize for Pedagogical Innovation as a team-member of the IMMUNOVA project (Active, interactive and flexible learning in immunology) led by Denis Hudrisier. For more details, watch the video (in French).T
10/2025
Our latest work on the role of Moesin during osteoclast fusion and function is highlighted in J Cell Biol:
https://rupress.org/jcb/article/224/11/e202509068/278400/Moesin-strikes-an-Actin-g-balance-to-regulate
and a press release has been edited by Inserm:
https://presse.inserm.fr/vieillissement-maladies-et-cancer-de-los-la-moesine-une-molecule-qui-pese-dans-la-balance/71346/
07/2024
In our article published in the EMBO Journal, we demonstrate that macrophage migration can occur without the ERM proteins, which were previously considered essential for any cell migration. Their results show that specific mechanisms are at work in these key cells of our immune system.
In French, from the CNRS and INSB “En direct des labos” : https://www.insb.cnrs.fr/fr/cnrsinfo/la-migration-des-macrophages-un-mecanisme-qui-deroge-la-regle
In English : http://10.58.64.217/the-migration-of-macrophages-a-mechanism-that-deviates-from-the-norm/
04/2023
Our work demonstrates how macrophage polarization drives their ability to fuse with #HIV-1 infected T cells via the CD81/RhoA-ROCK/Myosin axis published in J Cell Biol.
In French, from the CNRS and INSB « En direct des labos »:
https://www.insb.cnrs.fr/fr/cnrsinfo/infection-des-macrophages-par-les-lymphocytes-t-vih-fusionner-pour-mieux-infecter
https://www.cnrs.fr/endirectdeslabos/lettre.php?numero=339
Mojgan Naghavi also discussed thie paper in the J Cell Biol spotlight. Follow this link to read this article.
01/2021
English video version of our recent study describing how the tuberculosis bacillus takes advantage of host lipids to subvert the macrophage metabolic state published in Cell Reports.
English
Français
07/2020
Etudier la migration des macrophages dans les tumeurs (Fondation ARC – Projets soutenus)
05/2020
Siglec-1 : bridging the synergy beween Mycobacterium tuberculosis and HIV-1
05/2020
Bilan 2019 de l’INSB. « La co-infection VIH/Tuberculose à pleins tubes »
04/2019
CNRS News. « La co-infection VIH/tuberculose à pleins tubes »
03/2020
Ladepeche.fr (Accueil/Santé/Recherche médicale) : « Toulouse. Frédéric Lagarrigue traque les mauvaises cellules »
07/2019
Back Scatter (Physics today) : « Tunneling nanotubes connect diseases »


2025
Chen RH, Costa-Filho AJ, Debnath J, Galli T, Ge L, Goberdhan D, Guo W, He K, Jacob R, Kang T, Lee MG, Li L, Lolicato F, Lu J, Malhotra V, Nickel W, Nikoletopoulou V, Ogden SK, Sagia GM, Shao F, Shi A, Thery C, Vérollet C, Villeneuve J, Verweij F, Wang Y, Wang J, Wu S, Ye Y, Yin H, Yu L, Zhang M, Zhang Y, Zhou X, Zurzolo C. Beyond the Secretory Pathway: New Insights Into Protein Release. Traffic. 2025 Oct;26(10-12):e70022. doi: 10.1111/tra.70022. PMID: 41195561 Free PMC article. Review.
Rivault A, Bernut A, Ben-Neji M, Abrantes M, Jansen M, Huc-Brandt S, Besteiro S, Bordat Y, Nguyen-Chi M, Audemard N, Mesleard-Roux M, Perrais D, Neyrolles O, Lugo-Villarino G, Vérollet C, Espert L, Beaumelle B. HIV-1 Tat favors the multiplication of Mycobacterium tuberculosis and Toxoplasma by inhibiting clathrin-mediated endocytosis and autophagy. PLoS Pathog. 2025 Sep 11;21(9):e1013183. doi: 10.1371/journal.ppat.1013183. eCollection 2025 Sep. PMID: 40934260
Cervero P, Barger SR, Verdys P, Herzog R, Paul T, Palmieri M, Poincloux R, Linder S, Krendel M. Myo1e/f at the podosome base regulate podosome dynamics and promote macrophage migration. Eur J Cell Biol, 2025 Sep 1;104(4):151514. doi: https://doi.org/10.1101/2025.04.28.651090
Masson C, Scandola C, Rinckel JY, Proamer F, Janus-Bell E, Batool F, Osmani N, Goetz JG, Mallo L, Léon C, Bornert A, Poincloux R, Destaing O, Mansson A, Qian H, Lehmann M, Eckly A. Megakaryocytes assemble a three-dimensional cage of extracellular matrix that controls their maturation and anchoring to the vascular niche. Elife 2025 https://doi.org/10.7554/eLife.104963.1
2024
Cronin S, de Vries-Egan A, Vahlas Z, Czernikier A, Melucci C, Pereyra Gerber P, O’Neil T, Gloss B, Sharabas M, Turk G, Verollet C, Balboa L, Palmer S, Duette G. The immunosuppressive Tuberculosis-associated microenvironment inhibits viral replication and promotes HIV-1 latency in CD4+ T cells. iScience. 2024, 27(7):110324
Maire K, Chamy L, Ghazali S, Carratala-Lasserre M, Zahm M, Bouisset C, Métais A, Combes-Soia L, de la Fuente-Vizuete L, Trad H, Chaubet A, Savignac M, Gonzalez de Peredo A, Subramaniam A, Joffre O, Lutz P, Lamsoul I. Fine-tuning levels of filamins a and b as a specific mechanism sustaining Th2 lymphocyte functions. Nat Comm. 2024, 15, 10574
Verdys P, Rey Barroso J, Girel A, Vermeil J, Bergert M, Sanchez T, Métais A, Mangeat T, Bellard E, Bigot C, Astarie-Dequeker C, Labrousse A, Girard J-P, Maridonneau-Parini I, Vérollet C, Lagarrigue F, Diz-Muñoz A, Heuvingh J, Piel M, Du Roure O, Le Cabec V, Carréno S, Poincloux R. Ezrin, radixin, and Moesin are dispensable for macrophage migration and cellular cortex mechanics. EMBO J. 2024 doi: 10.1038/s44318-024-00173-7
Rey-Barroso J., O. Dufrançais O. and Vérollet C. Tunneling Nanotubes in Myeloid Cells: Perspectives for Health and Infectious Diseases. Springer Nature (Book Chapter 17), Results Cell Differentiation, 2024, 73:419-434. doi: 10.1007/978-3-031-62036-2_17. PMID: 39242388
Dufrancais O, Verdys P, Métais A, Juzans M, Sanchez T, Bergert M, Plozza M, Halper J, Panebianco CJ, Mascarau R, Gence R, Arnaud G, Ben Neji M, Maridonneau-Parini I, Le Cabec V, Boerckel JD, Pavlos NJ, Diz-Muñoz A, Lagarrigue F, Blin-Wakkach C, Carréno S, Poincloux R, Burkhardt JK, Raynaud-Messina B, Vérollet C. Moesin activation controls bone resorption and tunneling nanotube-dependent osteoclast fusion. bioRxiv [Preprint]. 2024 May 15:2024.05.13.593799. doi: 10.1101/2024.05.13.593799
Gilbert T, Gorlt C, Barbier M, Duployer B, Plozza M, Dufrancais O, Martet LE, Dalbard E, Segot L, Tenailleau C, Haren L, Vérollet C, Bierkamp C, Merdes A. Loss of ninein interferes with osteoclast formation and causes premature ossification. eLife. 2024. 13:e93457
Kumari R, Ven K, Chastney M, Peränen J, Aaron J, Almeida-Souza L, Kremneva E, Poincloux R, Chez TL, Gunning PW, Ivaska J, Lappalainen P. Specialized actin nanoscale layers control focal adhesion turnover. Nat. Comm. 2024. 15(1)2047
Lou E, Verollet C, Winkler F, Zurzolo C, Valdebenito-Silva S, Eugenin E. Tunneling nanotubes and tumor microtubes – Emerging data on their roles in intercellular communication and pathophysiology: Summary of an international FASEB Catalyst Conference October 2023. FASEB J. 2024. 38(5):e23514.
Faivre N, Verollet C, Dumas F. The chemokine receptor CCR5: multi-faceted hook for HIV-1. Retrovirology. 2024. 21(1):2.
Rey-Barroso J, Munaretto A, Rouquie N, Mougel A, Chassa M, Gadat S, Dewingle O, Poincloux R, Cadot S, Ysebaert L, Quillet-Mary A, Dupré L. Lymphocyte migration and retention properties affected by ibrutinib in chronic lymphocytic leukemia. Haematologica. 2024. 109(3):809-823
2023
Maio M, Joly M, Vahlas Z, Barros J, Marín Franco J, Genoula M, Monard S, Vecchione M, Fuentes F, Polo Virginia G, Quiroga M, Vermeulen M, Argüello RJ, Inwentarz S, Musella R, Ciallella L, Montaner P González, Palmero D, Villarino G, del Carmen Sasiain M, Neyrolles O, Verollet C*, Balboa L*,# Elevated glycolytic metabolism of monocytes limits the generation of HIF-1α-driven migratory dendritic cells in tuberculosis Elife. 2023 12:RP89319. https://doi.org/10.7554/eLife.89319.1
Mascarau R, B. Raynaud-Messina, C. Vérollet. Macrophage infection by fusion with HIV-1 infected lymphocytes: Catch meto fuse. Med Sci. Aug-Sep;39(8-9):602-605. doi: 10.1051/medsci/2023098. Epub 2023 Sep 11. PMID: 37695146 French
Affannoukoué K, S. Labouesse, G. Maire, I. Gallais, J. Savatier, M. Allain, M. Rasedujjaman, L. Legoff, J. Idier, R. Poincloux, F. Pelletier, C. Leterrier, T. Mangeat, A. Sentenac. Super-resolved total internal reflextion fluorescence microscopy using random illuminations, TIRF-RIM. Optica, 2023, 10(8) 1009-1017
Mascarau, R., M. Woottum, L. Fromont, R. Gence, V. Cantaloube-Ferrieu, Z. Vahlas, K. Lévêque, F. Bertrand, T. Beunon, A. Métais, H. El Costa, N. Jabrane-Ferrat, Y. Gallois, N. Guibert, J.-L. Davignon, G. Favre, I. Maridonneau-Parini, R. Poincloux, B. Lagane, S. Bénichou, B. Raynaud-Messina, and C. Vérollet. 2023. Productive HIV-1 infection of tissue macrophages by fusion with infected CD4+ T cells. J Cell Biol. 2023 (5) e202205103 – Among the eleven of the articles published in the last year that most interested our readers https://rupress.org/jcb/collection/22023/The-Year-in-Cell-Biology-2023?nbd=40786096593&nbd_source=campaigner&utm_source=Email_marketing&utm_campaign=YiCB_2023&cmp=1&utm_medium=HTMLEmail
2022
Dupont, M., S. Rousset, T.-P.V. Manh, S.C. Monard, K. Pingris, S. Souriant, Z. Vahlas, T. Velez, R. Poincloux, I. Maridonneau-Parini, O. Neyrolles, G. Lugo-Villarino, and C. Vérollet. 2022. Dysregulation of the IFN-I signaling pathway by Mycobacterium tuberculosis leads to exacerbation of HIV-1 infection of macrophages. J Leukoc Biol. 112:1329–1342.
Han, M., M. Woottum, R. Mascarau, Z. Vahlas, C. Verollet, and S. Benichou. 2022. Mechanisms of HIV-1 cell-to-cell transfer to myeloid cells. J Leukoc Biol. 112:1261–1271.
Jasnin, M., J. Hervy, S. Balor, A. Bouissou, A. Proag, R. Voituriez, J. Schneider, T. Mangeat, I. Maridonneau-Parini, W. Baumeister, S. Dmitrieff, and R. Poincloux. 2022. Elasticity of podosome actin networks produces nanonewton protrusive forces. Nat Commun. 13:3842.
Portes, M., T. Mangeat, N. Escallier, O. Dufrancais, B. Raynaud-Messina, C. Thibault, I. Maridonneau-Parini, C. Vérollet, and R. Poincloux. 2022. Nanoscale architecture and coordination of actin cores within the sealing zone of human osteoclasts. Elife. 11.
Corral D, Charton A, Krauss MZ, Blanquart E, Levillain F, Lefrançais E, Sneperger T, Vahlas Z, Girard JP, Eberl G, Poquet Y, Guéry JC, Argüello RJ, Belkaid Y, Mayer-Barber KD, Hepworth MR, Neyrolles O, Hudrisier D. ILC precursors differentiate into metabolically distinct ILC1-like cells during Mycobacterium tuberculosis infection. Cell Rep. 39(3):110715
Santoni, K., D. Pericat, L. Gorse, J. Buyck, M. Pinilla, L. Prouvensier, S. Bagayoko, A. Hessel, S.A. Leon-Icaza, E. Bellard, S. Mazères, E. Doz-Deblauwe, N. Winter, C. Paget, J.-P. Girard, C.T.N. Pham, C. Cougoule, R. Poincloux, M. Lamkanfi, E. Lefrançais, E. Meunier, and R. Planès. 2022. Caspase-1-driven neutrophil pyroptosis and its role in host susceptibility to Pseudomonas aeruginosa. PLoS Pathog. 18:e1010305.
2021
Benet, S., C. Gálvez, F. Drobniewski, I. Kontsevaya, L. Arias, M. Monguió-Tortajada, I. Erkizia, V. Urrea, R.-Y. Ong, M. Luquin, M. Dupont, J. Chojnacki, J. Dalmau, P. Cardona, O. Neyrolles, G. Lugo-Villarino, C. Vérollet, E. Julián, H. Furrer, H.F. Günthard, P.R. Crocker, G. Tapia, F.E. Borràs, J. Fellay, P.J. McLaren, A. Telenti, P.-J. Cardona, B. Clotet, C. Vilaplana, J. Martinez-Picado, and N. Izquierdo-Useros. 2021. Dissemination of Mycobacterium tuberculosis is associated to a SIGLEC1 null variant that limits antigen exchange via trafficking extracellular vesicles. J Extracell Vesicles. 10:e12046.
Bernard-Raichon, L., A. Colom, S.C. Monard, A. Namouchi, M. Cescato, H. Garnier, S.A. Leon-Icaza, A. Métais, A. Dumas, D. Corral, N. Ghebrendrias, P. Guilloton, C. Vérollet, D. Hudrisier, A. Remot, P. Langella, M. Thomas, C. Cougoule, O. Neyrolles, and G. Lugo-Villarino. 2021. A Pulmonary Lactobacillus murinus Strain Induces Th17 and RORγt(+) Regulatory T Cells and Reduces Lung Inflammation in Tuberculosis. J Immunol. 207:1857–1870.
Dufrançais, O., R. Mascarau, R. Poincloux, I. Maridonneau-Parini, B. Raynaud-Messina, and C. Vérollet. 2021. Cellular and molecular actors of myeloid cell fusion: podosomes and tunneling nanotubes call the tune. Cell Mol Life Sci. 78:6087–6104.
Gelis, A., J. Morel, B. Amara, C. Mauri, H. Rouays, C. Verollet, I. Almeras, N. Frasson, A. Dupeyron, I. Laffont, J.-P. Daures, and C. Herlin. 2021. “Doctor, how long will it take?” Results from an historical cohort on surgical pressure ulcer healing delay and related factors in persons with spinal cord injury. J Tissue Viability. 30:237–243.
Mangeat, T., S. Labouesse, M. Allain, A. Negash, E. Martin, A. Guénolé, R. Poincloux, C. Estibal, A. Bouissou, S. Cantaloube, E. Vega, T. Li, C. Rouvière, S. Allart, D. Keller, V. Debarnot, X.B. Wang, G. Michaux, M. Pinot, R. Le Borgne, S. Tournier, M. Suzanne, J. Idier, and A. Sentenac. 2021. Super-resolved live-cell imaging using random illumination microscopy. Cell Rep Methods. 1:100009.
Pires, D., M. Calado, T. Velez, M. Mandal, M.J. Catalão, O. Neyrolles, G. Lugo-Villarino, C. Vérollet, J.M. Azevedo-Pereira, and E. Anes. 2021. Modulation of Cystatin C in Human Macrophages Improves Anti-Mycobacterial Immune Responses to Mycobacterium tuberculosis Infection and Coinfection With HIV. Front Immunol. 12:742822.
Tertrais, M., C. Bigot, E. Martin, R. Poincloux, A. Labrousse, and I. Maridonneau-Parini. 2021. Phagocytosis is coupled to the formation of phagosome-associated podosomes and a transient disruption of podosomes in human macrophages. Eur J Cell Biol. 100:151161.
2020
Accarias, S., T. Sanchez, A. Labrousse, M. Ben-Neji, A. Boyance, R. Poincloux, I. Maridonneau-Parini, and V. Le Cabec. 2020. Genetic engineering of Hoxb8-immortalized hematopoietic progenitors – a potent tool to study macrophage tissue migration. J Cell Sci. 133:jcs236703.
Dupont, M., G. Lugo-Villarino, and C. Vérollet. 2020a. [The Siglec-1 receptor: bridging the infectious synergy between Mycobacterium tuberculosis and HIV-1]. Med Sci (Paris). 36:855–858.
Dupont, M., S. Souriant, L. Balboa, T.-P. Vu Manh, K. Pingris, S. Rousset, C. Cougoule, Y. Rombouts, R. Poincloux, M. Ben Neji, C. Allers, D. Kaushal, M.J. Kuroda, S. Benet, J. Martinez-Picado, N. Izquierdo-Useros, M.D.C. Sasiain, I. Maridonneau-Parini, O. Neyrolles, C. Vérollet, and G. Lugo-Villarino. 2020b. Tuberculosis-associated IFN-I induces Siglec-1 on tunneling nanotubes and favors HIV-1 spread in macrophages. Elife. 9.
Genoula, M., J.L. Marín Franco, M. Maio, B. Dolotowicz, M. Ferreyra, M.A. Milillo, R. Mascarau, E.J. Moraña, D. Palmero, M. Matteo, F. Fuentes, B. López, P. Barrionuevo, O. Neyrolles, C. Cougoule, G. Lugo-Villarino, C. Vérollet, M.D.C. Sasiain, and L. Balboa. 2020. Fatty acid oxidation of alternatively activated macrophages prevents foam cell formation, but Mycobacterium tuberculosis counteracts this process via HIF-1α activation. PLoS Pathog. 16:e1008929.
Guérit, D., P. Marie, A. Morel, J. Maurin, C. Verollet, B. Raynaud-Messina, S. Urbach, and A. Blangy. 2020. Primary myeloid cell proteomics and transcriptomics: importance of β-tubulin isotypes for osteoclast function. J Cell Sci. 133.
Marín Franco, J.L., M. Genoula, D. Corral, G. Duette, M. Ferreyra, M. Maio, M.B. Dolotowicz, O.E. Aparicio-Trejo, E. Patiño-Martínez, A. Charton, A. Métais, F. Fuentes, V. Soldan, E.J. Moraña, D. Palmero, M. Ostrowski, P. Schierloh, C. Sánchez-Torres, R. Hernández-Pando, J. Pedraza-Chaverri, Y. Rombouts, D. Hudrisier, E. Layre, C. Vérollet, I. Maridonneau-Parini, O. Neyrolles, M.D.C. Sasiain, G. Lugo-Villarino, and L. Balboa. 2020. Host-Derived Lipids from Tuberculous Pleurisy Impair Macrophage Microbicidal-Associated Metabolic Activity. Cell Rep. 33:108547
Mascarau, R., F. Bertrand, A. Labrousse, I. Gennero, R. Poincloux, I. Maridonneau-Parini, B. Raynaud-Messina, and C. Vérollet. 2020. HIV-1-Infected Human Macrophages, by Secreting RANK-L, Contribute to Enhanced Osteoclast Recruitment. Int J Mol Sci. 21.
2019
van den Dries, K., S. Linder, I. Maridonneau-Parini, and R. Poincloux. 2019. Probing the mechanical landscape – new insights into podosome architecture and mechanics. J Cell Sci. 132:jcs236828.
Lugo-Villarino, G., C. Cougoule, E. Meunier, Y. Rombouts, C. Vérollet, and L. Balboa. 2019. Editorial: The Mononuclear Phagocyte System in Infectious Disease. Front Immunol. 10:1443.
Morel, J., C. Herlin, B. Amara, C. Mauri, H. Rouays, C. Verollet, I. Almeras, N. Frasson, A. Dupeyron, C. Jourdan, J.-P. Daures, and A. Gelis. 2019. Risk factors of pelvic pressure ulcer recurrence after primary skin flap surgery in people with spinal cord injury. Ann Phys Rehabil Med. 62:77–83.
Raynaud-Messina, B., C. Verollet, and I. Maridonneau-Parini. 2019. The osteoclast, a target cell for microorganisms. Bone. 127:315–323.
Souriant, S., L. Balboa, M. Dupont, K. Pingris, D. Kviatcovsky, C. Cougoule, C. Lastrucci, A. Bah, R. Gasser, R. Poincloux, B. Raynaud-Messina, T. Al Saati, S. Inwentarz, S. Poggi, E.J. Moraña, P. González-Montaner, M. Corti, B. Lagane, I. Vergne, C. Allers, D. Kaushal, M.J. Kuroda, M.D.C. Sasiain, O. Neyrolles, I. Maridonneau-Parini, G. Lugo-Villarino, and C. Vérollet. 2019. Tuberculosis Exacerbates HIV-1 Infection through IL-10/STAT3-Dependent Tunneling Nanotube Formation in Macrophages. Cell Rep. 26:3586-3599
Souriant, S., M. Dupont, O. Neyrolles, I. Maridonneau-Parini, G. Lugo-Villarino, and C. Vérollet. 2019. [Tunneling nanotube formation in HIV-1-infected human macrophages: building bridges for efficient HIV-1 dissemination during co-infection with Mycobacterium tuberculosis]. Med Sci (Paris). 35:825–827.
Xie, M., H. Leroy, R. Mascarau, M. Woottum, M. Dupont, C. Ciccone, A. Schmitt, B. Raynaud-Messina, C. Vérollet, J. Bouchet, L. Bracq, and S. Benichou. 2019. Cell-to-Cell Spreading of HIV-1 in Myeloid Target Cells Escapes SAMHD1 Restriction. mBio. 10.
2018
Baschieri, F., S. Dayot, N. Elkhatib, N. Ly, A. Capmany, K. Schauer, T. Betz, D.M. Vignjevic, R. Poincloux, and G. Montagnac. 2018. Frustrated endocytosis controls contractility-independent mechanotransduction at clathrin-coated structures. Nat Commun. 9:3825.
Cervero, P., C. Wiesner, A. Bouissou, R. Poincloux, and S. Linder. 2018. Lymphocyte-specific protein 1 regulates mechanosensory oscillation of podosomes and actin isoform-based actomyosin symmetry breaking. Nat Commun. 9:515.
Colado, A., M. Genoula, C. Cougoule, J.L. Marín Franco, M.B. Almejún, D. Risnik, D. Kviatcovsky, E. Podaza, E.E. Elías, F. Fuentes, I. Maridonneau-Parini, F.R. Bezares, H. Fernandez Grecco, M. Cabrejo, C. Jancic, M.D.C. Sasiain, M. Giordano, R. Gamberale, L. Balboa, and M. Borge. 2018. Effect of the BTK inhibitor ibrutinib on macrophage- and γδ T cell-mediated response against Mycobacterium tuberculosis. Blood Cancer J. 8:100.
Cougoule, C., C. Lastrucci, R. Guiet, R. Mascarau, E. Meunier, G. Lugo-Villarino, O. Neyrolles, R. Poincloux, and I. Maridonneau-Parini. 2018. Podosomes, But Not the Maturation Status, Determine the Protease-Dependent 3D Migration in Human Dendritic Cells. Front Immunol. 9:846.
Desvignes, E., A. Bouissou, A. Laborde, T. Mangeat, A. Proag, C. Vieu, C. Thibault, I. Maridonneau-Parini, and R. Poincloux. 2018. Nanoscale Forces during Confined Cell Migration. Nano Lett. 18:6326–6333.
Dupont, M., S. Souriant, G. Lugo-Villarino, I. Maridonneau-Parini, and C. Vérollet. 2018. Tunneling Nanotubes: Intimate Communication between Myeloid Cells. Front Immunol. 9:43.
Genoula, M., J.L. Marín Franco, M. Dupont, D. Kviatcovsky, A. Milillo, P. Schierloh, E.J. Moraña, S. Poggi, D. Palmero, D. Mata-Espinosa, E. González-Domínguez, J.C. León Contreras, P. Barrionuevo, B. Rearte, M.O. Córdoba Moreno, A. Fontanals, A. Crotta Asis, G. Gago, C. Cougoule, O. Neyrolles, I. Maridonneau-Parini, C. Sánchez-Torres, R. Hernández-Pando, C. Vérollet, G. Lugo-Villarino, M.D.C. Sasiain, and L. Balboa. 2018. Formation of Foamy Macrophages by Tuberculous Pleural Effusions Is Triggered by the Interleukin-10/Signal Transducer and Activator of Transcription 3 Axis through ACAT Upregulation. Front Immunol. 9:459.
Gonçalves-de-Albuquerque, C.F., I. Rohwedder, A.R. Silva, A.S. Ferreira, A.R.M. Kurz, C. Cougoule, S. Klapproth, T. Eggersmann, J.D. Silva, G.P. de Oliveira, V.L. Capelozzi, G.G. Schlesinger, E.R. Costa, R. de C.E. Estrela Marins, A. Mócsai, I. Maridonneau-Parini, B. Walzog, P.R. Macedo Rocco, M. Sperandio, and H.C. de Castro-Faria-Neto. 2018. The Yin and Yang of Tyrosine Kinase Inhibition During Experimental Polymicrobial Sepsis. Front Immunol. 9:901.
Gui, P., M. Ben-Neji, E. Belozertseva, F. Dalenc, C. Franchet, J. Gilhodes, A. Labrousse, E. Bellard, M. Golzio, R. Poincloux, I. Maridonneau-Parini, and V. Le Cabec. 2018. The Protease-Dependent Mesenchymal Migration of Tumor-Associated Macrophages as a Target in Cancer Immunotherapy. Cancer Immunol Res. 6:1337–1351.
Le Cabec, V., E. Van Goethem, R. Guiet, and I. Maridonneau-Parini. 2011. [Phagocyte migration: an overview]. Med Sci (Paris). 27:1112–1119.
2017
Bouissou, A., A. Proag, N. Bourg, K. Pingris, C. Cabriel, S. Balor, T. Mangeat, C. Thibault, C. Vieu, G. Dupuis, E. Fort, S. Lévêque-Fort, I. Maridonneau-Parini, and R. Poincloux. 2017. Podosome Force Generation Machinery: A Local Balance between Protrusion at the Core and Traction at the Ring. ACS Nano. 11:4028–4040.
Demy, D.L., M. Tauzin, M. Lancino, V. Le Cabec, M. Redd, E. Murayama, I. Maridonneau-Parini, N. Trede, and P. Herbomel. 2017. Trim33 is essential for macrophage and neutrophil mobilization to developmental or inflammatory cues. J Cell Sci. 130:2797–2807.
Troegeler, A., I. Mercier, C. Cougoule, D. Pietretti, A. Colom, C. Duval, T.-P. Vu Manh, F. Capilla, R. Poincloux, K. Pingris, J. Nigou, J. Rademann, M. Dalod, F.A.W. Verreck, T. Al Saati, G. Lugo-Villarino, B. Lepenies, D. Hudrisier, and O. Neyrolles. 2017. C-type lectin receptor DCIR modulates immunity to tuberculosis by sustaining type I interferon signaling in dendritic cells. Proc Natl Acad Sci U S A. 114:E540–E549.
2016
Proag, A., A. Bouissou, C. Vieu, I. Maridonneau-Parini, and R. Poincloux. 2016. Evaluation of the force and spatial dynamics of macrophage podosomes by multi-particle tracking. Methods. 94:75–84.
2015
Alvarez-Zarate, J., H.L. Matlung, T. Matozaki, T.W. Kuijpers, I. Maridonneau-Parini, and T.K. van den Berg. 2015. Regulation of Phagocyte Migration by Signal Regulatory Protein-Alpha Signaling. PLoS One. 10:e0127178.
Balboa, L., J. Barrios-Payan, E. González-Domínguez, C. Lastrucci, G. Lugo-Villarino, D. Mata-Espinoza, P. Schierloh, D. Kviatcovsky, O. Neyrolles, I. Maridonneau-Parini, C. Sánchez-Torres, M. del C. Sasiain, and R. Hernández-Pando. 2015. Diverging biological roles among human monocyte subsets in the context of tuberculosis infection. Clin Sci (Lond). 129:319–330.
Lastrucci, C., V. Baillif, A. Behar, T. Al Saati, M. Dubourdeau, I. Maridonneau-Parini, and C. Cougoule. 2015. Molecular and cellular profiles of the resolution phase in a damage-associated molecular pattern (DAMP)-mediated peritonitis model and revelation of leukocyte persistence in peritoneal tissues. FASEB J. 29:1914–1929.
Lastrucci, C., A. Bénard, L. Balboa, K. Pingris, S. Souriant, R. Poincloux, T. Al Saati, V. Rasolofo, P. González-Montaner, S. Inwentarz, E.J. Moraña, I. Kondova, F.A.W. Verreck, M. del C. Sasiain, O. Neyrolles, I. Maridonneau-Parini, G. Lugo-Villarino, and C. Cougoule. 2015. Tuberculosis is associated with expansion of a motile, permissive and immunomodulatory CD16(+) monocyte population via the IL-10/STAT3 axis. Cell Res. 25:1333–1351.
Medina, I., C. Cougoule, M. Drechsler, B. Bermudez, R.R. Koenen, J. Sluimer, I. Wolfs, Y. Döring, V. Herias, M. Gijbels, I. Bot, S. de Jager, C. Weber, J. Cleutjens, T.J.C. van Berkel, K.-J. Sikkink, A. Mócsai, I. Maridonneau-Parini, O. Soehnlein, and E.A.L. Biessen. 2015. Hck/Fgr Kinase Deficiency Reduces Plaque Growth and Stability by Blunting Monocyte Recruitment and Intraplaque Motility. Circulation. 132:490–501.
Proag, A., A. Bouissou, T. Mangeat, R. Voituriez, P. Delobelle, C. Thibault, C. Vieu, I. Maridonneau-Parini, and R. Poincloux. 2015. Working together: spatial synchrony in the force and actin dynamics of podosome first neighbors. ACS Nano. 9:3800–3813.
Vérollet, C., V. Le Cabec, and I. Maridonneau-Parini. 2015. HIV-1 Infection of T Lymphocytes and Macrophages Affects Their Migration via Nef. Front Immunol. 6:514.
Vérollet, C., S. Souriant, E. Bonnaud, P. Jolicoeur, B. Raynaud-Messina, C. Kinnaer, I. Fourquaux, A. Imle, S. Benichou, O.T. Fackler, R. Poincloux, and I. Maridonneau-Parini. 2015. HIV-1 reprograms the migration of macrophages. Blood. 125:1611–1622.
Vérollet, C., S. Souriant, B. Raynaud-Messina, and I. Maridonneau-Parini. 2015. [HIV-1 drives the migration of macrophages]. Med Sci (Paris). 31:730–733.
2014
Bouissou, A., C. Vérollet, H. de Forges, L. Haren, Y. Bellaïche, F. Perez, A. Merdes, and B. Raynaud-Messina. 2014. γ-Tubulin Ring Complexes and EB1 play antagonistic roles in microtubule dynamics and spindle positioning. EMBO J. 33:114–128.
Gouzy, A., G. Larrouy-Maumus, D. Bottai, F. Levillain, A. Dumas, J.B. Wallach, I. Caire-Brandli, C. de Chastellier, T.-D. Wu, R. Poincloux, R. Brosch, J.-L. Guerquin-Kern, D. Schnappinger, L.P. Sório de Carvalho, Y. Poquet, and O. Neyrolles. 2014. Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathog. 10:e1003928.
Gui, P., A. Labrousse, E. Van Goethem, A. Besson, I. Maridonneau-Parini, and V. Le Cabec. 2014. Rho/ROCK pathway inhibition by the CDK inhibitor p27(kip1) participates in the onset of macrophage 3D-mesenchymal migration. J Cell Sci. 127:4009–4023.
Labernadie, A., A. Bouissou, P. Delobelle, S. Balor, R. Voituriez, A. Proag, I. Fourquaux, C. Thibault, C. Vieu, R. Poincloux, G.M. Charrière, and I. Maridonneau-Parini. 2014. Protrusion force microscopy reveals oscillatory force generation and mechanosensing activity of human macrophage podosomes. Nat Commun. 5:5343.
Maridonneau-Parini, I. 2014. Podosomes are disrupted in PAPA syndrome. Blood. 123:2597–2599.
Maridonneau-Parini, I. 2014. Control of macrophage 3D migration: a therapeutic challenge to limit tissue infiltration. Immunol Rev. 262:216–231.
Park, H., A. Dovas, S. Hanna, C. Lastrucci, C. Cougoule, R. Guiet, I. Maridonneau-Parini, and D. Cox. 2014. Tyrosine phosphorylation of Wiskott-Aldrich syndrome protein (WASP) by Hck regulates macrophage function. J Biol Chem. 289:7897–7906.
Troegeler, A., C. Lastrucci, C. Duval, A. Tanne, C. Cougoule, I. Maridonneau-Parini, O. Neyrolles, and G. Lugo-Villarino. 2014. An efficient siRNA-mediated gene silencing in primary human monocytes, dendritic cells and macrophages. Immunol Cell Biol. 92:699–708.
Wiesner, C., V. Le-Cabec, K. El Azzouzi, I. Maridonneau-Parini, and S. Linder. 2014. Podosomes in space: macrophage migration and matrix degradation in 2D and 3D settings. Cell Adh Migr. 8:179–191.
2013
Ben Amara, A., L. Gorvel, K. Baulan, J. Derain-Court, C. Buffat, C. Vérollet, J. Textoris, E. Ghigo, F. Bretelle, I. Maridonneau-Parini, and J.-L. Mege. 2013. Placental macrophages are impaired in chorioamnionitis, an infectious pathology of the placenta. J Immunol. 191:5501–5514.
Bochet, L., C. Lehuédé, S. Dauvillier, Y.Y. Wang, B. Dirat, V. Laurent, C. Dray, R. Guiet, I. Maridonneau-Parini, S. Le Gonidec, B. Couderc, G. Escourrou, P. Valet, and C. Muller. 2013. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 73:5657–5668.
Marchetti, M., D. Capela, R. Poincloux, N. Benmeradi, M.-C. Auriac, A. Le Ru, I. Maridonneau-Parini, J. Batut, and C. Masson-Boivin. 2013. Queuosine biosynthesis is required for sinorhizobium meliloti-induced cytoskeletal modifications on HeLa Cells and symbiosis with Medicago truncatula. PLoS One. 8:e56043.
Vérollet, C., A. Gallois, R. Dacquin, C. Lastrucci, S.N.M. Pandruvada, N. Ortega, R. Poincloux, A. Behar, C. Cougoule, C. Lowell, T. Al Saati, P. Jurdic, and I. Maridonneau-Parini. 2013. Hck contributes to bone homeostasis by controlling the recruitment of osteoclast precursors. FASEB J. 27:3608–3618.
2012
Ariel, A., I. Maridonneau-Parini, P. Rovere-Querini, J.S. Levine, and H. Mühl. 2012. Macrophages in inflammation and its resolution. Front Immunol. 3:324.
Bouchet, J., C. Hérate, C.A. Guenzel, C. Vérollet, A. Järviluoma, J. Mazzolini, S. Rafie, P. Chames, D. Baty, K. Saksela, F. Niedergang, I. Maridonneau-Parini, and S. Benichou. 2012. Single-domain antibody-SH3 fusions for efficient neutralization of HIV-1 Nef functions. J Virol. 86:4856–4867.
Cougoule, C., E. Van Goethem, V. Le Cabec, F. Lafouresse, L. Dupré, V. Mehraj, J.-L. Mège, C. Lastrucci, and I. Maridonneau-Parini. 2012. Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3D environments. Eur J Cell Biol. 91:938–949.
Guiet, R., C. Vérollet, I. Lamsoul, C. Cougoule, R. Poincloux, A. Labrousse, D.A. Calderwood, M. Glogauer, P.G. Lutz, and I. Maridonneau-Parini. 2012. Macrophage mesenchymal migration requires podosome stabilization by filamin A. J Biol Chem. 287:13051–13062.
2011
Botella, H., P. Peyron, F. Levillain, R. Poincloux, Y. Poquet, I. Brandli, C. Wang, L. Tailleux, S. Tilleul, G.M. Charrière, S.J. Waddell, M. Foti, G. Lugo-Villarino, Q. Gao, I. Maridonneau-Parini, P.D. Butcher, P.R. Castagnoli, B. Gicquel, C. de Chastellier, and O. Neyrolles. 2011. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 10:248–259.
Guiet, R., E. Van Goethem, C. Cougoule, S. Balor, A. Valette, T. Al Saati, C.A. Lowell, V. Le Cabec, and I. Maridonneau-Parini. 2011. The process of macrophage migration promotes matrix metalloproteinase-independent invasion by tumor cells. J Immunol. 187:3806–3814.
Hawkins, R.J., R. Poincloux, O. Bénichou, M. Piel, P. Chavrier, and R. Voituriez. 2011. Spontaneous contractility-mediated cortical flow generates cell migration in three-dimensional environments. Biophys J. 101:1041–1045.
Labrousse, A.M., E. Meunier, J. Record, A. Labernadie, A. Beduer, C. Vieu, T. Ben Safta, and I. Maridonneau-Parini. 2011. Frustrated phagocytosis on micro-patterned immune complexes to characterize lysosome movements in live macrophages. Front Immunol. 2:51.
Le Cabec, V., E. Van Goethem, R. Guiet, and I. Maridonneau-Parini. 2011. [Phagocyte migration: an overview]. Med Sci (Paris). 27:1112–1119.
Lugo-Villarino, G., C. Vérollet, I. Maridonneau-Parini, and O. Neyrolles. 2011. Macrophage polarization: convergence point targeted by mycobacterium tuberculosis and HIV. Front Immunol. 2:43.
Poincloux, R., O. Collin, F. Lizárraga, M. Romao, M. Debray, M. Piel, and P. Chavrier. 2011. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc Natl Acad Sci U S A. 108:1943–1948.
Van Goethem, E., R. Guiet, S. Balor, G.M. Charrière, R. Poincloux, A. Labrousse, I. Maridonneau-Parini, and V. Le Cabec. 2011. Macrophage podosomes go 3D. Eur J Cell Biol. 90:224–236.
Vérollet, C., G.M. Charrière, A. Labrousse, C. Cougoule, V. Le Cabec, and I. Maridonneau-Parini. 2011. Extracellular proteolysis in macrophage migration: losing grip for a breakthrough. Eur J Immunol. 41:2805–2813.
2010
Boissan, M., O. De Wever, F. Lizarraga, D. Wendum, R. Poincloux, N. Chignard, C. Desbois-Mouthon, S. Dufour, B. Nawrocki-Raby, P. Birembaut, M. Bracke, P. Chavrier, C. Gespach, and M.-L. Lacombe. 2010. Implication of metastasis suppressor NM23-H1 in maintaining adherens junctions and limiting the invasive potential of human cancer cells. Cancer Res. 70:7710–7722.
Cougoule, C., V. Le Cabec, R. Poincloux, T. Al Saati, J.-L. Mège, G. Tabouret, C.A. Lowell, N. Laviolette-Malirat, and I. Maridonneau-Parini. 2010. Three-dimensional migration of macrophages requires Hck for podosome organization and extracellular matrix proteolysis. Blood. 115:1444–1452.
Labernadie, A., C. Thibault, C. Vieu, I. Maridonneau-Parini, and G.M. Charrière. 2010. Dynamics of podosome stiffness revealed by atomic force microscopy. Proc Natl Acad Sci U S A. 107:21016–21021.
Van Goethem, E., R. Poincloux, F. Gauffre, I. Maridonneau-Parini, and V. Le Cabec. 2010. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol. 184:1049–1061.
Vérollet, C., Y.M. Zhang, V. Le Cabec, J. Mazzolini, G. Charrière, A. Labrousse, J. Bouchet, I. Medina, E. Biessen, F. Niedergang, S. Bénichou, and I. Maridonneau-Parini. 2010. HIV-1 Nef triggers macrophage fusion in a p61Hck- and protease-dependent manner. J Immunol. 184:7030–7039.
Add Your Heading Text Here
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
2026.
Galveston, Texas, USA
Christel Verollet, Gordon conference “Bone and Teeth” , Invited lecture
2025.
Heidelberg, Germany
Christel Verollet, DGZ International meeting, invited lecture
Lyon
Renaud Poincloux, Symposium on Monocytes and Macrophages, Lyon (invited lecture)
Nara, Japan
Renaud Poincloux, NAIST (invited lecture)
Toulouse, PhD Pub, June 10th
Marianna Plozza, live at the “bar L’autruche” for scientific popularization
Suzhou, China
Christel Verollet-Roullet, invited lecture, Cold Spring Harbor Asia meeting China
Online
Renaud Poincloux, Immunobiophysics semina series, invited lecture
2024.
Christel Verollet-Roullet, invited talk, CONICET/IMEX, mid-term evaluation of the IRP
Rome, Italy
Christel Verollet-Roullet, selected talk, HIV Workshop
Oxford, UK
Christel Verollet-Roullet, invited talk, International Musculoskeletal Biology symposium
Perpignan
Renaud Poincloux, invited talk, 20th meeting of the Canceropole GSO
Toulouse
Renaud Poincloux, invited talk, Les Avancées Scientifiques de la Recherche Biomédicale Toulousaine
Toulouse
Renaud Poincloux, invited talk, Institut Clément Ader
Toronto, Canada
Renaud Poincloux, invited talk, SickKids Research Institute
2023.
Presqu’île de Giens / 16 Nov.
Renaud Poincloux is co-organizing a cell biology + microscopy meeting for the SBCF @ MiFoBio
Barcelona, Spain / 26 Oct
Christel Verollet-Roullet, invited talk, Hot Topic HIV Seminar
Faseb Conferences On-Line / 5 Oct
Christel Vérollet-Roullet, invited talk, Tunneling Nanotubes
Institut Cochin / 27 Jun
Christel Vérollet sera membre du jury d’HDR du Dr Nikaïa Smith.
Waterville Valley, USA / 5 Jun.
Christel Vérollet will give a talk at @ Phagocyte Gordon conference
Sarah Monard will give a talk at @ Phagocyte Gordon conference
Ile d’Oléron / 11 May
Renaud Poincloux is selected to speak @ the AQV days
Liverpool, UK / 14 Apr.
Christel Vérollet is invited to speak and Ophélie Dufrançais is selected to speak @ ECTS
Paris / 13 Apr.
Renaud Poincloux will be in the PhD jury of Magdalena Kopec @ PMMH ESPCI
Paris / 31 Jan.
Christel Vérollet is invited to speak @ ANRS TB
Nice / 19 Jan.
Christel Vérollet gives a seminar @ LP2M, Université Côte d’Azur, Nice
2022.
Paris / 13 Dec.
Renaud Poincloux is co-organizing the NewMic4CellBio meeting for the GDR AQV @IJM
Paris / 21 Nov.
Christel Vérollet is in the PhD jury of Mingyu Han @ Institut Cochin
Paris / 16 Nov.
Christel Vérollet is in the PhD jury of Victor Garcia @ Imagine
Paris / 15 Nov.
Zoï Vahlas has a selected presentation @ ANRS
Aarhus, Denmark / 4 Nov.
Renaud Poincloux gives a seminar @ iNano
Perth, Australia / 3 Nov.
Christel Vérollet gives a seminar @ University of Western Australia
Copenhagen, Denmark / 1 Nov.
Renaud Poincloux gives a seminar @ Copenhagen University
Sète / 4-7 Oct.
Renaud Poincloux and Ophélie Lefrançais are co-organizing the 8th Meeting of The Invadosome Consortium “Pushing the frontiers of cell adhesion and invasion”
Paris / 13 Apr.
Renaud Poincloux will be in the PhD jury of Paul Rivier @ IAB Grenoble
Toulouse / 5 July
Rémi Mascarau is selected to present his work @ MicrobiOccitanie 2022
Toulouse / 1 June
Renaud Poincloux is invited to give a seminar @ l’Académie des Sciences et Belles Lettres de Toulouse
La Baule / 1 June
Ophélie Dufrançais is selected to present her work @ Journées Françaises de Biologie des Tissus Minéralisés
Montpellier / 25 March
Christel Vérollet gives a seminar @ IRIM
Toulouse / 22-24 Feb.
Christel Vérollet will speak @ the 6th Global Forum on TB Vaccines
Paris / 19-21 Jan.
Renaud Poincloux participated in the organisation of AQV days 2022 : quantitative approaches to living systems @ Institut Jacques Monod
2021.
Toulouse / 10 Dec.
Véronique Le Cabec is invited to speak at the 3rd “Avancées en imagerie du vivant en Occitanie” @ CBI
Orsay / 27 Oct.
Renaud Poincloux is invited to give a seminar @ LPTMS
Lyon / 21 Sept.
Christel Vérollet was in the PhD jury of Marion Delphin @ CIRI
Online conference / 4 May
Christel Vérollet is invited to speak @ Laboratoire Pathologies, Imagerie et Biothérapies Oro-faciales, UPR2496, Paris
Online conference / 16 Apr.
Renaud Poincloux is invited to speak @ ENS/ESPCI biophysics seminars
Online conference / 18 March
Rémi Mascarau speaks @ ANRS AC41 International Symposium
Online conference / 24-26 March
Isabelle Maridonneau-Parini is invited to speak @ the EMBO Workshop ImmunoBioPhysics
Online conference / 23 March
Renaud Poincloux co-organizes the AQV Meets Idylle webinar
Online conference / 20-22 Jan.
Renaud Poincloux co-organizes the AQV days 2021: Quantitative approaches to living systems
2020.
Online conference / 16-18 Nov.
Renaud Poincloux is a jury member for prizes @ the SFBD meeting: From cells to embryo
Paris / 5 Nov.
Christel Vérollet was in the HDR jury of Mickael Menager @ Imagine
Online conference / 21 Oct.
Christel Vérollet speaks @ ANRS AC41 International Symposium
Toulouse / 3-5 May
Claire Bigot is co-organizing the Ph.D Student Days of the BSB Doctoral School @ Toulouse University Auditorium
Toulouse / 25 March
Véronique Le Cabec is invited to speak @ Workshop “Matrice extracellulaire et cancer”, Cancéropole Grand Sud-Ouest
2019.
Toulouse / 10 Dec.
Renaud Poincloux is co-organizing the 2nd “Avancées en imagerie du vivant en Occitanie” @ Hôtel de Région
Toulouse / 3-4 Dec.
Frédéric Lagarrigue is invited to speak @ 7ème journée du club français sur les plaquettes et les mégacaryocytes.
Toulouse / 11 Nov.
Isabelle Maridonneau-Parini will give a seminar @ Académie des Sciences, Inscription et Belles Lettres
Toulouse / 8 Nov.
Rémi Mascarau is presenting a poster and Javier Rey Barroso is chair @ le symposium des étudiants, IPBS
Marseille / 6- 8 Nov.
Véronique Le Cabec is invited to speak @ les 41es Journées de la Société Française de Sénologie et de Pathologie Mammaire, Palais du Pharo
Presqu-île de Giens / 5-7 Nov.
Renaud Poincloux is selected to talk @ CellTiss Days 2019
Lyon / 4-6 Nov.
Christel Vérollet is selected to talk @ 10th Imaging the Cell
Toulouse / 19 Sept.
Christel Vérollet will give a seminar @ CPTP
Toulouse / 4 July
Frédéric Lagarrigue will meet donators of La Fondation Toulouse Cancer Santé @ Musee des Abattoirs
Londres, UK / 19-22 June
Isabelle Maridonneau-Parini is invited to talk and Renaud Poincloux is selected for a short talk @ Integrated mechano-chemical signals in invasion symposium
Palaiseau / 21 May
Isabelle Maridonneau-Parini is giving a seminar @ Immuno-mechanics day, Ecole Polytechnique
Rotterdam, the Netherlands / 15-18 May
Frédéric Lagarrigue and Isabelle Maridonneau-Parini are selected to speak @ the 6th Zoo meeting: Cell Adhesion and Migration in Inflammation and Cancer
Toulouse / 6 May
Isabelle Maridonneau-Parini is giving a seminar @ IMRCP
Oxford, UK / 1-5 Apr.
Maeva Dupont is presenting a poster @ EMBO workshop on Pathogen Immunity and Signalling
Lyon / 28 March
Isabelle Maridonneau-Parini is speaking @ Cancer cells on chip 2
Grenoble / 18-19 March
Isabelle Maridonneau-Parini is invited to talk @ Cell migration : Swimming versus Crawling, DysCo federation
Toulouse / 14 March
Frédéric Lagarrigue will present his project to donators of La Fondation Toulouse Cancer Santé @ CRCT
Montpellier / 18-20 Feb.
Maeva Dupont will present her project @ MicrobioOccitanie
Toulouse / 15 Feb.
Frédéric Lagarrigue will present his project to donators of La Fondation Toulouse Cancer Santé @ CRCT
Toulouse / 4 Feb.
Frédéric Lagarrigue will meet donators of La Fondation Toulouse Cancer Santé @ Toulouse Casino
Barcelona, Spain / 24th Jan.
Christel Vérollet is invited to talk @ IrsiCaixa
2018.
Toulouse / 4 Dec.
Renaud Poincloux is co-organising a symposium on Advances in Life Imaging (and gives a seminar) @ Museum d’Histoire Naturelle
Toulouse / 30 Nov.
Solène Accarias gave a seminar, chaired by Christel Vérollet !, and received a price !! @ the 2nd Symposium of the Occitanie Network Monocytes-Macrophages
Santiago de Chile, Chile / 13-17 Nov.
Isabelle Maridonneau-Parini is invited to talk @ SlamTB
Buenos Aires, Argentina / 12 Nov.
Christel Vérollet will give a talk @ CONICET
Toulouse / 19 Oct.
Véronique Le Cabec will give a seminar @ StromaLab
Seignosse / 5-12 Oct.
Natacha Escallier & Renaud Poincloux are organising a workshop on DONALD nanoscopy @ MiFoBio Seignosse (2018 Oct. 5-12)
Toulouse / 4 Oct.
Marion Portes and Maeva Dupont will present their project @ the 3rd PhD student symposium, IPBS
Toulouse / 11&12 July
Renaud Poincloux is co-animating a workshop on microscopy for kids @ ESOF – Place du Capitole
Barcelona, Spain / 30 May – 1st June
Isabelle Maridonneau-Parini is invited to talk @ the 52nd ESCI
Toulouse / 3 May
Christel Verollet is invited to speak @ the IPBS-CPTP day
Toulouse / 5 Apr.
Marion Portes will present her project @ les Journées de l’Ecole Doctorale
Toulouse / 29 March
Isabelle Maridonneau-Parini will talk about (iPSC)-derived macrophages @ le Club Macrophage de Toulouse
Monaco / 23 March
Christel Verollet is giving a talk @ the 20th Journées Française de Biologie des Tissus Déminéralisés
Jouy-en-Josas / 15 March
Renaud Poincloux is invited to speak @ INRA
Frankfurt, Germany / 8 March
Isabelle Maridonneau-Parini is invited to talk @ Goethe University
Paris / 9 Feb.
Shanti Souriant is presenting her work @ the 4th Sidaction Scientific Day
2017.
Helsinki, Finland / 12 Dec.
Renaud Poincloux is invited to speak @ the Cell Challenge symposium
Toulouse / 27 Nov.
Véronique Le Cabec is giving a talk @ IUCT
Toulouse / 21 Nov.
Christel Vérollet speaks @ the 6th FRBT day
Montpellier / 20 Nov.
Céline Cougoule will co-organise the first Symposium of the Occitanie network of Monocytes-Macrophages
Lyon / 18-20 May
Brigitte Raynaud-Messina is presenting a poster @ les Journées Françaises de Biologie des Tissus Minéralisés
Lisbon, Portugal / 9-15 July
Isabelle Maridonneau-Parini will be present @ the 17th HFSP Awardees Meeting
Waterville Valley, NH, USA / 11-17 June
Isabelle Maridonneau-Parini is invited to speak @ the Gordon Research Conference on phagocytes
Helsinki, Finland / 7 June
Renaud Poincloux is giving a talk @ the European Cytoskeleton Forum
Bordeaux / 11 Apr
Renaud Poincloux is giving a talk @ Focus On Microscopy
Toulouse / 06 Jan
Céline Cougoule will defend her Habilitation à diriger des recherches @ the LCC
2016.
Toulouse / 07 Dec.
Renaud Poincloux will defend his Habilitation à diriger des recherches @ the IPBS
Toulouse / 24 Nov.
Renaud Poincloux is giving a talk @ the FRBT day
Toulouse / 22 Nov.
Renaud Poincloux will meet sponsors for la Fondation Toulouse Cancer @ le Musée des Abattoirs
Toulouse / 18 Nov.
Anais Bouissou is speaking @ 1st Symposium of the Club Macrophage Toulouse “Macrophage diversity in health and diseases”, co-organised by Céline Cougoule
Toulouse / 14 Nov.
Shanti Souriant has an interview with Ensemble, the newspaper from Sidaction (printed in 95,000 copies)
Arcachon / 7-9 Nov.
Renaud Poincloux is giving a talk @ les Journées du GDR CellTiss 2016
Nantes / 28 Sept.
Véronique Le Cabec is giving a talk @ Niches & Cancer 2016
Roma, Italy / 24 Sept.
Shanti Souriant and Christel Vérollet are giving talks @ the HIV Workshop XII
Naxos, Italy / 22 Sept.
Véronique Le Cabec is giving a talk @ 100 years of phagocytes / Cell symposium
Paris / 19 Sept.
Shanti Souriant has a poster @ the EMBO Conference Tuberculosis 2016
Erlangen, Germany / 12-14 Sept.
Shanti Souriant is presenting her work @ Frontiers of Retrovirology Conference 2016
Cambridge, UK / 22 June
Renaud Poincloux is giving a talk @ the European Cytoskeleton Forum
Trondheim, Norway / 10 June
Renaud Poincloux is an invited speaker @ the SCANDEM meeting
Paris / 27-29 Apr.
Véronique Le Cabec is giving a talk @ the Phagocyte worshop, ESCI
Zacatecas, Mexico / 19-23 Apr.
Céline Cougoule is an invited speaker @ el XXII Congreso Nacional de Inmunologia
Paris / 7 Apr.
Shanti Souriant is giving a talk @ the ANRS
Toulouse / 3-5 Feb.
Véronique Le Cabec is giving a talk @ the Oncoweek
Toulouse / 25 Jan.
Véronique Le Cabec is giving a talk @ IUCT
La Rochelle / 20-22 Jan.
Véronique Le Cabec is an invited speaker @ the 1st congress of GDR 3697 MICRONIT
2015.
Hinxton, UK / 7-8 Dec.
Edgardo Ferran is a co-organizer and speaker in the EMBL-EBI Industry Workshop on “Quantitative Systems Pharmacology II” @ the European Bioinformatics Institute (EBI)
Bordeaux / 30 Oct.
Isabelle Maridonneau-Parini is giving a seminar @ la Plateforme de Génomique Fonctionnelle
Toulouse / 24 Nov.
Renaud Poincloux is giving a talk @ the 4th FRBT annual meeting
Oaxtepec, Mexico / 10-13 Nov.
Céline Cougoule is an invited speaker @ el XXIV Foro Nacional de Investigación en Salud – IMSS
Budapest, Hungary / 11-12 Nov.
Arnaud Labrousse is giving a talk @ the Tarkinaid Consortium meeting
Tucuman, Argentina / 4 Nov.
Edgardo Ferran is an invited speaker @ BioArgentina NOA organized by the Argentinian Chamber of Biotecnology (CAB), Tucuman, Argentina
Paris / 30 Oct.
Isabelle Maridonneau-Parini is giving a seminar @ the Pasteur Institute
St Paul de Vence / 16-20 Oct.
Isabelle Maridonneau-Parini is an invited speaker and chairwoman and Renaud Poincloux is presenting a poster @ the Integrated mechano-chemical signals in invasion congress
Buenos Aires, Argentina / 14 Oct.
Edgardo Ferran is an invited speaker @ the Argentinian Conference on Bioinformatics and Computational Biology (CAB2C)
Buenos Aires, Argentina / 8-9 Oct.
Edgardo Ferran is a chairman, co-organizer and speaker @ the EMBL-MinCyT industry workshop on “Biotechnology and Genomics in Livestock, Agriculture and Human Health”.
Cargèse / 28 Sep.-2 Oct.
Isabelle Maridonneau-Parini is an invited speaker @ the Quantitative Biology of Signaling congress
Toulouse / 4 June
Edgardo Ferran is giving a seminar @ IPBS
Barcelona, Spain / 13-15 May
Amsha Proag is presenting a poster @ the 6th European Cell Mechanics meeting
Marseille / 6- 7 May
Céline Cougoule is presenting a poster @ the 4th MycoClub Conference
Paris / 8 Apr.
Isabelle Maridonneau-Parini is giving a seminar @ Centre de Recherche sur l’Inflammation, Bichat
Toulouse / 2 Apr.
Shanti Souriant is presenting a poster @ les Journées de l’école doctorale BSB
Boston, USA / 31 Mar – 1 Apr
Edgardo Ferran is a speaker @ the EMBL-EBI Industry workshop on “Immunogenomics”
Boston, USA / 30 Mar
Edgardo Ferran is giving a seminar @ Genzyme
Hinxton, UK / 18-19 Mar.
Edgardo Ferran is a co-organizer of the EMBL-EBI Industry Workshop on “Connectivity Map and LINC” @ the European Bioinformatics Institute
Barcelona, Spain / 3 Feb.
Edgardo Ferran is giving two seminars @ the CRG
2014.
Nantes / 13-14 Nov.
Céline Cougoule is giving a course on latest developments in inflammation research @ the EUmBRella meeting
Lille / 4-6 Nov.
Isabelle Maridonneau-Parini is an invited speaker @ the French Society for Immunology Annual meeting
Sopron, Hungary / 6-8 Oct.
Céline Cougoule is giving a talk @ the Tarkinaid Consortium meeting
Toulouse / 3 Oct.
Isabelle Maridonneau-Parini is giving a seminar @ la FRBT
Toulouse / 26 June
Véronique Le Cabec is presenting her work @ the Workshop on 3D models and applications in oncology, Canceropole GSO
Marseille / 15-16 May
Anaïs Bouissou is giving a seminar @ the 4th French Cell Adhesion Club Symposium
Limoges / 14-16 May
Christel Vérollet is presenting a poster @ la Société Française du Tissu minéralisé
Montpellier / 27-28 Apr.
Christel Vérollet and Shanti Souriant are presenting a poster @ the ANRS symposium
Z








Alumni
- Océane DEWINGLE: PhD (2025), still in the lab as a post-doc
- Natacha FAIVRE: PhD (2025), soon working for Vibiosphen
- Thibaut SANCHEZ: PhD (2023), currently postdoctoral fellow at the Institute for Regeneration and Repair (IRR) – University of Edinburgh, Scotland
- Claire BIGOT: PhD (2023), currently Researcher at Physiogenex, Escalquens, France
- Ophélie DUFRANCAIS : PhD (2023), currently Local Sales Representative for MedChemExpress (Toulouse, France)
- Perrine VERDYS: PhD (2023), currently postdoctoral fellow at the Center for Cancer Immune Therapy, Denmark
- Zoï VALHAS: postdoctoral fellow, currently Team Leader at Evotec (Toulouse, France)
- Rémi MASCARAU: PhD (2022), currently postdoctoral fellow at the Pasteur Intitute at Lille (France)
- Isabelle MARIDONNEAU-PARINI: Principal Investigator, currently retired (France)
- Natacha ESCALLIER: Research Assistant
- Margot TERTRAIS: Postdoctoral fellow, currently at STROMALab (Toulouse, France)
- Frédéric LAGARRIGUE: postdoctoral fellow, currently Atip-Avenir team leader at IPBS (Toulouse, France)
- Solène ACCARIAS: Postdoctoral fellow
- Myriam BENNEJI: Research Assistant, currently at IPBS (Toulouse, France)
- Marion PORTES: PhD student (2019)
- Maéva DUPONT: PhD student (2019), currently a postdoctoral fellow at Oxford University (UK)
- Karine PINGRIS: research assitant, currently at Evotec (Toulouse, France)
- Shanti SOURIANT: PhD student (2017), currently at Police Nationale (Toulouse, France)
- Anaïs BOUISSOU: Postdoctoral fellow, currently at Police Nationale (Toulouse, France)
- Annie BEHAR: Research Assistant
- Céline COUGOULE: CR CNRS, currently at IPBS (Toulouse, France)
- Claire LASTRUCCI: PhD student (2014), currently Scientific Illustrator and 3D Animator (Barcelona, Spain)
- Philippe GUI: PhD student (2014), currently a postdoctoral fellow at CRG (Barcelona, Spain)
- Anna LABERNADIE: PhD student (2012), currently a postdoctoral fellow at IBEC (Barcelona, Spain)
- Romain GUIET: PhD student (2011), currently an engineer at EPFL (Lausanne, Switzerland)
- Amsha PROAG: Postdoctoral fellow, currently at Thalès (Toulouse, France)
- Guillaume CHARRIERE: Postdoctoral fellow, currently Associate Professor (Montpellier, France)
- Emeline VAN GOETHEM: PhD student (2010), currently at Ambiotis (Toulouse, France)
- Claire VINCENT: PhD (2007)
- Jérôme CASTANDET: PhD (2005)
- Christel VILLENEUVE: PhD (2004)
- Catherine ASTARIE: CR CNRS, currently at IPBS (Toulouse, France)
- Federica PIMPINELLI: Postdoctoral fellow, currently pharmacist (Milan)
- Sébastien CARRENO: PhD student (2001), currently group leader at IRIC (Montreal, Canada)
- Heidi WELCH: PhD student, currently group leader at Babraham Institute (Cambridge, UK)
Teaching
Fabrice Dumas is associate professor at the Sciences and Engineering Faculty. He teaches 200 hours of fundamental biochemistry and membrane dynamics to first year-students (lectures, exercises and practical courses) as well as graduate students.
Arnaud Labrousse is associate professor at the Sciences and Engineering Faculty. He teaches 200 hours of fundamental cell biology, trafficking, cell signaling and imaging to second and third year-students (lectures, exercises and practical courses) as well as graduate students.
Christel Vérollet & Véronique Le Cabec are giving a yearly 4-hour class on Innate Immunity for graduate students in the 2nd year of Master of Immunology, Infection and Inflammation.



