Interactions des mycobactéries avec les cellules hôtes

Responsable d'équipe
L’objectif principal de notre groupe de recherche est de mieux comprendre les interactions hôte-pathogène dans la tuberculose aux niveaux moléculaire et cellulaire, de la découverte de nouveaux gènes de virulence chez M. tuberculosis à l’identification des mécanismes immunitaires impliqués dans la défense de l’hôte contre le pathogène, avec l’objectif de proposer de meilleures stratégies pour contrôler la maladie.
Nous étudions les interactions hôte-pathogène dans la tuberculose, avec un intérêt particulier pour l'immunité antituberculeuse et pour les liens qui unissent évolution, physiologie et virulence chez M. tuberculosis.

Notre recherche vise à explorer les liens entre le métabolisme et la virulence chez le bacille de la tuberculose, Mycobacterium tuberculosis, et comment ce lien a été façonné au cours de l’évolution. En particulier, nous utilisons le profilage de l’expression génique globale (RNA-seq) et le criblage de bibliothèques de mutants de transposition (Tn-seq) pour identifier les gènes et les voies jouant un rôle clé dans les interactions hôte-pathogène dans la tuberculose. Nous avons notamment identifié un nouveau mécanisme de contrôle immunitaire inné des pathogènes par l’intoxication au zinc, et des stratégies de résistance chez les mycobactéries pathogènes impliquant des P-ATPases. Nous cherchons actuellement à comprendre la fonction de plusieurs P-ATPases dans la physiologie et la virulence de M. tuberculosis. Nous utilisons également le dual RNA-seq et l’analyse Tn-seq pour comprendre l’adaptation de M. tuberculosis à divers stress imposés par l’hôte, notamment l’hypoxie.
Notre laboratoire s’intéresse à la compréhension de l’immunité contre la tuberculose en l’absence et en présence d’une co-infection avec le virus du sida VIH-1. Dans ce contexte, nous étudions le rôle des récepteurs innés, en particulier les lectines de type C, et des glycanes exprimés par les cellules hôtes dans l’immunité anti-mycobactérienne. Par exemple, nous avons récemment découvert que la lectine de type C DCIR module l’immunité contre la tuberculose en soutenant la signalisation de l’interféron de type I dans les cellules dendritiques. Les mécanismes moléculaires impliqués dans la signalisation de DCIR sont en cours de décryptage grâce à des expériences biochimiques et (phospho)protéomiques.
Nous explorons également le rôle joué par les lymphocytes et les cellules lymphoïdes innées (ILC) dans l’immunité contre la tuberculose. La contribution des ILC à la réponse immunitaire contre M. tuberculosis est mal connue et nos données récentes indiquent que l’infection par la TB induit une certaine plasticité phénotypique liée au métabolisme dans le compartiment des ILC, conférant une protection précoce contre M. tuberculosis. En ce qui concerne les autres lymphocytes, nous développons actuellement des approches d’imagerie et des approches fonctionnelles pour comprendre quelles sous-populations de lymphocytes T ont le meilleur accès, la meilleure interaction et les meilleures propriétés fonctionnelles pour atteindre les cellules infectées par M. tuberculosis.
Enfin, dans le contexte de la co-infection TB/VIH, une hypothèse prédominante pour expliquer l’exacerbation de l’infection par le VIH-1 est que l’infection des macrophages par M. tuberculosis module les environnements inflammatoires locaux en faveur de la réplication du VIH-1. Nous exploitons actuellement différentes approches in vitro pour mimer cet effet bystander émanant des sites d’infection par M. tuberculosis afin de disséquer les mécanismes moléculaires et cellulaires rendant les monocytes et macrophages humains sensibles à l’infection par le VIH-1. Parallèlement, dans le contexte d’une co-infection in vivo, nous établissons des corrélations entre nos résultats in vitro et des lésions pulmonaires de primates non humains et des échantillons humains, notamment des sérums et du liquide d’épanchement pleural. Dans l’ensemble, notre objectif ultime est de proposer de nouvelles cibles à potentiel diagnostique et thérapeutique contre la comorbidité établie entre le SIDA et la tuberculose.
Équipe
Chercheurs-Enseignants chercheurs
Claude Gutierrez (PR-Émérite, UPS)
Denis Hudrisier (PR CE, UPS)
Geanncarlo Lugo (DR2, CNRS)
Fabien Létisse (PR, UPS)
Olivier Neyrolles (DR CE, CNRS)
Yannick Poquet (MCU HC, UPS)
Yoann Rombouts (CR, CNRS)
Ingénieures
Nelly Gilles
Florence Levillain (IE, CNRS)
Bertille Voisin
Post-doctorants
Saurabh Chugh
Pierre Dupuy
Marion Faucher
Doctorants
Louis Benastre
Maxime Caouaille
Natacha Faivre
Wendy Le Mouellic
Sarah Monard
Stella Rousset
Nos projets de recherche (en anglais)
Boudehen*, Faucher* et al. (2022) Mycobacterial resistance to zinc poisoning requires assembly of P-ATPase-containing membrane metal efflux platforms. Nat Commun
Corral et al. (2022) ILC precursors differentiate into metabolically distinct ILC1-like cells during Mycobacterium tuberculosis infection. Cell Rep
Souriant et al. (2019) Tuberculosis exacerbates HIV-1 infection through IL-10/STAT3-dependent tunneling nanotube formation in macrophages. Cell Rep
Troegeler et al. (2017) C-type lectin receptor DCIR modulates immunity to tuberculosis by sustaining type I interferon signaling in dendritic cells. Proc Natl Acad Sci USA
Freire*, Gutierrez* et al. (2019) An NAD+ phosphorylase toxin triggers Mycobacterium tuberculosis cell death. Mol Cell
Levillain*, Poquet* et al. (2017) Horizontal acquisition of a hypoxia-responsive molybdenum cofactor biosynthesis pathway contributed to Mycobacterium tuberculosis pathoadaptation. PLOS Pathog
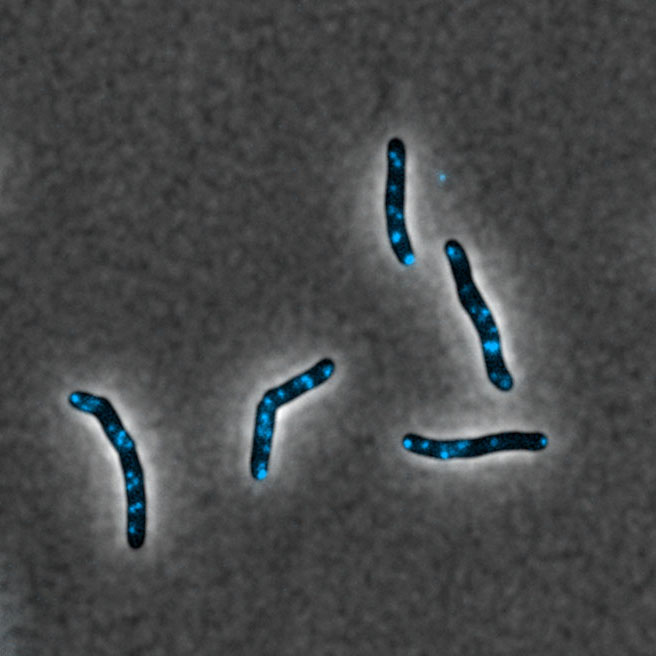
Imagerie par microscopie confocale de microdomaines membranaires fonctionnels (bleu) chez Mycobacterium tuberculosis © JM Boudehen
Prizes
October 2021 – Olivier Neyrolles is awarded the CNRS silver medal. Read more… (in French)
November 2020 – Olivier Neyrolles is awarded the Jacques Piraud Prize from the Fondation pour la Recherche Médicale.
October 2018 – The 2018 PEPS Prize for Pedagogical Innovation is awarded to the IMMUNOVA project (Active, interactive and flexible learning in immunology) led by Denis Hudrisier.
The IMMUNOVA project is composed of two axes: the first is the strong use of digital resources allowing the students to prepare themselves before and after the face-to-face sessions (also available online), which provides greater flexibility and learning autonomy for all students, especially those who cannot attend classes according to established schedules; for the second axis, the students are offered to conduct team projects in immunology according to different approaches (e.g., digital resource generation, interviews with researchers, 3D printing of proteins in FabLabs, conception of serious games).
Known more about the project – Watch the video! (in French)
December 2017 – Anthony Troegeler is awarded the Bretesche Prize in medicine from the Toulouse Academy of sciences.
Anthony did his PhD studies in our lab where he worked on the role of the innate immune receptor DCIR in immunity to tuberculosis. His work was published in January 2017 in Proc Natl Acad Sci USA. He will receive the Prize on December 3 at the Hôtel d’Assézat, Toulouse.
June 2017 – Yoann Rombouts is awarded the IGO Young Glycoscientist Award 2017.
December 2016 – Olivier Neyrolles receives the Sanofi-Institut Pasteur National Junior Award in the field of neglected tropical diseases for his research into tuberculosis, during a ceremony at the Institut Pasteur, Paris. Olivier and his team contributed to understanding several aspects of the complex interactions between the tuberculosis bacillus, Mycobacterium tuberculosis, and its host, including anti-mycobacterial immunity and mycobacterial evolution, physiology and virulence…
December 2014 – Alexandre Gouzy receives the Sanofi 2014 Prize from the Toulouse Academy of Sciences.
February 2014 – Olivier Neyrolles receives the “Coup d’élan pour la Recherche Française” Prize from the Bettencourt Schueller Foundation during a ceremony at the Institut de France, Paris, in which Madame F. Bettencourt Meyers presented the Prize. This Prize rewards teams specifically chosen for the promising nature of their research programs. The Prize recognises both the quality of the work of Olivier Neyrolles’ team and the quality of the research conducted in the Tuberculosis & Infection Biology Department at IPBS in the field of vaccine and antibiotics development.
April 2012 – Hélène Botella, winner of the AXA-Academy of Sciences prize based on her work in the involvement of zinc as a natural mechanism to fight against infections.
2009 – Olivier Neyrolles receives the CNRS Bronze medal.
Press Releases
How the tuberculosis bacillus takes advantage of the lipids of its host
Siglec-1: Bridging the synergy between Mycobacterium tuberculosis and HIV-1
How the tuberculosis bacillus learned to respire in the absence of oxygen
A new target to fight tuberculosis: a population of immune cells favourable to the resilience of the bacillus… Press release in french
Collaborations
We are indebtful to our many national and international collaborators, including:
- Joel Ernst University of California, San Francisco, CA, USA
- Sho Yamasaki Research Institute for Microbial Diseases, Osaka, Japan
- Elsa Anes University of Lisbon, Portugal
- David Sancho National Cardiovascular Research Centre, Madrid, Spain
- Patrice Catty Commissariat à l’Energie Atomique, Grenoble, France
- Brigitte Gicquel, Ludovic Tailleux, Roland Brosch, Pierre Charneau & Lluis Quintana-Murci Institut Pasteur, Paris
- Matthias Willmans EMBL-Centre for Cellular and Molecular Imaging, Hamburg, Germany
- Manfred Wuhrer Leiden University Medical Centre, Leiden, The Netherlands
- Marcelo Kuroda University of California, Davis, CA, USA
- Luciana Balboa & Maria Carmen Sasiain Academia Nacional de Medicina, Buenos Aires, Argentina
- Luiz Pedro de Carvalho The Francis Crick Institute, London, UK
Funding
Our team is supported by several grants and fellowships from:
- European Union (FP7, H2020, Horizon Europe)
- ERA-NET JPI-AMR
- European Respiratory Society
- MSDAVENIR
- NIH
- Agence Nationale de la Recherche
- Agence Nationale de la Recherche sur le SIDA et les hépatites virales
- Fondation pour la Recherche Médicale
- Agence nationale de recherche sur le sida et les hépatites virales
- Fondation Bettencourt Schueller
- Fondation ARC pour la recherche sur le cancer
- Vaincre la Mucoviscidose
- Association Gregory Lemarchal
- Fondation Mérieux
- ECOS-Sud exchange programme (Argentina)
Our team is officially labelled by the Fondation pour la Recherche Médicale (2016-2019 and 2021-2024).
The complete list of our publications is available through Pubmed.
TB vaccine research
Bettencourt, P.J.G., Joosten, S.A., Lindestam Arlehamn, C.S., Behr, M.A., Locht, C., Neyrolles, O. (2021) 100 years of the Bacillus Calmette-Guerin vaccine. Vaccine 39, 7221-7222 (View)
Levillain, F.*, Kim, H.*, Woong Kwon, K., Clark, S., Cia, F., Malaga, W., Lanni, F., Brodin, P., Gicquel, B., Guilhot, C., Bancroft, G.J., Williams, A., Jae Shin, S., Poquet, Y.**, Neyrolles, O.** (2020) Preclinical assessment of a new live attenuated Mycobacterium tuberculosis Beijing-based vaccine for tuberculosis. Vaccine 38, 1416-1423 (View)
Voss, G., Casimiro, D., Neyrolles, O., Williams, A., Kaufmann, S.H.E., McShane, H., Hatherill, M., Fletcher, H.A. (2018) Progress and challenges in TB vaccine development. F1000Res 7, 199 (View)
Kaufmann, S.H.E., Dockrell, H.M., Drager, N., Ho, M.M., McShane, H., Neyrolles, O., Ottenhoff, T.H.M., Patel, B., Roordink, D., Spertini, F., Stenger, S., Thole, J., Verreck, F.A.W., Williams, A., Consortium, T. (2017) TBVAC2020: Advancing Tuberculosis Vaccines from Discovery to Clinical Development. Front Immunol 8, 1203 (View)
Scriba, T.J., Kaufmann, S.H., Henri Lambert, P., Sanicas, M., Martin, C., Neyrolles, O. (2016) Vaccination Against Tuberculosis With Whole-Cell Mycobacterial Vaccines. J Infect Dis 214, 659-664 (View)
Immunity to TB, TB/HIV coinfection and other pathogens
Maio, M., Joly, M., Vahlas, Z., Barros, J., Marín Franco, J.L., Genoula, M., Monard, S., Vecchione, M.B., Fuentes, F., Gonzalez Polo, V., Quiroga, M.F., Vermeulen, M., Argüello, R.J., Inwentarz, S., Musella, R., Ciallella, L., González Montaner, P., Palmero, D., Lugo Villarino, G., Sasiain, M.C., Neyrolles O., Verollet, C., Balboa, L. (2023) Elevated glycolytic metabolism of monocytes limits the generation of HIF-1α-driven migratory dendritic cells in tuberculosis. Elife (View)
Corral, D., Charton, A., Krauss, M.Z., Blanquart, E., Levillain, F., Lefrancais, E., Sneperger, T., Vahlas, Z., Girard, J.P., Eberl, G., Poquet, Y., Guery, J.C., Arguello, R.J., Belkaid, Y., Mayer-Barber, K.D., Hepworth, M.R., Neyrolles, O.**, Hudrisier, D.** (2022) ILC precursors differentiate into metabolically distinct ILC1-like cells during Mycobacterium tuberculosis infection. Cell Rep 39, 110715 (View)
Bernard-Raichon, L., Colom, A., Monard, S.C., Namouchi, A., Cescato, M., Garnier, H., Leon-Icaza, S.A., Metais, A., Dumas, A., Corral, D., Ghebrendrias, N., Guilloton, P., Verollet, C., Hudrisier, D., Remot, A., Langella, P., Thomas, M., Cougoule, C.**, Neyrolles, O.**, Lugo-Villarino, G**. (2021) A Pulmonary Lactobacillus murinus Strain Induces Th17 and RORgammat(+) Regulatory T Cells and Reduces Lung Inflammation in Tuberculosis. J Immunol 207, 1857-1870 (View)
Marin Franco, J.L., Genoula, M., Corral, D., Duette, G., Ferreyra, M., Maio, M., Dolotowicz, M.B., Aparicio-Trejo, O.E., Patino-Martinez, E., Charton, A., Metais, A., Fuentes, F., Soldan, V., Morana, E.J., Palmero, D., Ostrowski, M., Schierloh, P., Sanchez-Torres, C., Hernandez-Pando, R., Pedraza-Chaverri, J., Rombouts, Y., Hudrisier, D., Layre, E., Verollet, C., Maridonneau-Parini, I., Neyrolles, O., Sasiain, M.D.C., Lugo-Villarino, G., Balboa, L. (2020) Host-Derived Lipids from Tuberculous Pleurisy Impair Macrophage Microbicidal-Associated Metabolic Activity. Cell Rep 33, 108547 (View)
Dupont, M.*, Souriant, S.*, Balboa, L., Vu Manh, T.P., Pingris, K., Rousset, S., Cougoule, C., Rombouts, Y., Poincloux, R., Ben Neji, M., Allers, C., Kaushal, D., Kuroda, M.J., Benet, S., Martinez-Picado, J., Izquierdo-Useros, N., Sasiain, M.D.C., Maridonneau-Parini, I.**, Neyrolles, O.**, Verollet, C.**, Lugo-Villarino, G**. (2020) Tuberculosis-associated IFN-I induces Siglec-1 on tunneling nanotubes and favors HIV-1 spread in macrophages. Elife 9 (View)
Souriant, S., Balboa, L., Dupont, M., Pingris, K., Kviatcovsky, D., Cougoule, C., Lastrucci, C., Bah, A., Gasser, R., Poincloux, R., Raynaud-Messina, B., Al Saati, T., Inwentarz, S., Poggi, S., Morana, E.J., Gonzalez-Montaner, P., Corti, M., Lagane, B., Vergne, I., Allers, C., Kaushal, D., Kuroda, M.J., Sasiain, M.D.C., Neyrolles, O.**, Maridonneau-Parini, I.**, Lugo-Villarino, G.**, Verollet, C**. (2019) Tuberculosis Exacerbates HIV-1 Infection through IL-10/STAT3-Dependent Tunneling Nanotube Formation in Macrophages. Cell Rep 26, 3586-3599 e3587 (View) | This article was highlighted in Science
Dumas, A., Corral, D., Colom, A., Levillain, F., Peixoto, A., Hudrisier, D., Poquet, Y., Neyrolles, O. (2018) The Host Microbiota Contributes to Early Protection Against Lung Colonization by Mycobacterium tuberculosis. Front Immunol 9, 2656 (View)
Lugo-Villarino, G., Troegeler, A., Balboa, L., Lastrucci, C., Duval, C., Mercier, I., Benard, A., Capilla, F., Al Saati, T., Poincloux, R., Kondova, I., Verreck, F.A.W., Cougoule, C., Maridonneau-Parini, I., Sasiain, M.D.C., Neyrolles, O. (2018) The C-Type Lectin Receptor DC-SIGN Has an Anti-Inflammatory Role in Human M(IL-4) Macrophages in Response to Mycobacterium tuberculosis. Front Immunol 9, 1123 (View)
Benard, A., Sakwa, I., Schierloh, P., Colom, A., Mercier, I., Tailleux, L., Jouneau, L., Boudinot, P., Al-Saati, T., Lang, R., Rehwinkel, J., Loxton, A.G., Kaufmann, S.H.E., Anton-Leberre, V., O’Garra, A., Sasiain, M.D.C., Gicquel, B., Fillatreau, S.**, Neyrolles, O.**, Hudrisier, D.** (2018) B Cells Producing Type I IFN Modulate Macrophage Polarization in Tuberculosis. Am J Respir Crit Care Med 197, 801-813 (View)
Troegeler, A., Mercier, I., Cougoule, C., Pietretti, D., Colom, A., Duval, C., Vu Manh, T.P., Capilla, F., Poincloux, R., Pingris, K., Nigou, J., Rademann, J., Dalod, M., Verreck, F.A., Al Saati, T., Lugo-Villarino, G., Lepenies, B., Hudrisier, D., Neyrolles, O. (2017) C-type lectin receptor DCIR modulates immunity to tuberculosis by sustaining type I interferon signaling in dendritic cells. Proc Natl Acad Sci USA 114, E540-E549 (View)
Lastrucci, C., Benard, A., Balboa, L., Pingris, K., Souriant, S., Poincloux, R., Al Saati, T., Rasolofo, V., Gonzalez-Montaner, P., Inwentarz, S., Morana, E.J., Kondova, I., Verreck, F.A., Sasiain Mdel, C., Neyrolles, O.**, Maridonneau-Parini, I.**, Lugo-Villarino, G.**, Cougoule, C.** (2015) Tuberculosis is associated with expansion of a motile, permissive and immunomodulatory CD16(+) monocyte population via the IL-10/STAT3 axis. Cell Res 25, 1333-1351 (View)
Troegeler, A., Lastrucci, C., Duval, C., Tanne, A., Cougoule, C., Maridonneau-Parini, I.**, Neyrolles, O.**, Lugo-Villarino, G.** (2014) An efficient siRNA-mediated gene silencing in primary human monocytes, dendritic cells and macrophages. Immunol Cell Biol 92, 699-708
Lefevre, L.*, Lugo-Villarino, G.*, Meunier, E.*, Valentin, A., Olagnier, D., Authier, H., Duval, C., Dardenne, C., Bernad, J., Lemesre, J.L., Auwerx, J., Neyrolles, O., Pipy, B., Coste, A. (2013) The C-type lectin receptors dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity 38, 1038-1049 (View)
Tanne, A., Ma, B., Boudou, F., Tailleux, L., Botella, H., Badell, E., Levillain, F., Taylor, M.E., Drickamer, K., Nigou, J., Dobos, K.M., Puzo, G., Vestweber, D., Wild, M.K., Marcinko, M., Sobieszczuk, P., Stewart, L., Lebus, D., Gicquel, B., Neyrolles, O. (2009) A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med 206, 2205-2220 (View)
Tailleux, L., Pham-Thi, N., Bergeron-Lafaurie, A., Herrmann, J.L., Charles, P., Schwartz, O., Scheinmann, P., Lagrange, P.H., de Blic, J., Tazi, A., Gicquel, B., Neyrolles, O. (2005) DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med 2, e381 (View)
Tailleux, L., Schwartz, O., Herrmann, J.L., Pivert, E., Jackson, M., Amara, A., Legres, L., Dreher, D., Nicod, L.P., Gluckman, J.C., Lagrange, P.H., Gicquel, B., Neyrolles, O. (2003) DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med 197, 121-127 (View)
Maeda, N., Nigou, J., Herrmann, J.L., Jackson, M., Amara, A., Lagrange, P.H., Puzo, G., Gicquel, B., Neyrolles, O. (2003) The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J Biol Chem 278, 5513-5516 (View)
Mycobacterial evolution, physiology and virulence
Dupuy, P., Gutierez, C., Neyrolles, O. (2023) Modulation of bacterial membrane proteins activity by clustering into plasma membrane nanodomains. Mol Microbiol (View)
Boudehen, Y.M.*, Faucher, M.*, Marechal, X., Miras, R., Rech, J., Rombouts, Y., Seneque, O., Wallat, M., Demange, P., Bouet, J.Y., Saurel, O., Catty, P., Gutierrez, C., Neyrolles, O. (2022) Mycobacterial resistance to zinc poisoning requires assembly of P-ATPase-containing membrane metal efflux platforms. Nat Commun 13, 4731 (View)
Freire, D.M.*, Gutierrez, C.*, Garza-Garcia, A., Grabowska, A.D., Sala, A.J., Ariyachaokun, K., Panikova, T., Beckham, K.S.H., Colom, A., Pogenberg, V., Cianci, M., Tuukkanen, A., Boudehen, Y.M., Peixoto, A., Botella, L., Svergun, D.I., Schnappinger, D., Schneider, T.R., Genevaux, P., de Carvalho, L.P.S., Wilmanns, M., Parret, A.H.A.**, Neyrolles, O.** (2019) An NAD(+) Phosphorylase Toxin Triggers Mycobacterium tuberculosis Cell Death. Mol Cell 73, 1282-1291 e1288 (View)
Levillain, F.*, Poquet, Y.*, Mallet, L., Mazeres, S., Marceau, M., Brosch, R., Bange, F.C., Supply, P., Magalon, A., Neyrolles, O. (2017) Horizontal acquisition of a hypoxia-responsive molybdenum cofactor biosynthesis pathway contributed to Mycobacterium tuberculosis pathoadaptation. PLoS Pathog 13, e1006752 (View)
Neyrolles, O., Wolschendorf, F., Mitra, A., Niederweis, M. (2015) Mycobacteria, metals, and the macrophage. Immunol Rev 264, 249-263 (View)
Gouzy, A., Poquet, Y., Neyrolles, O. (2014) Nitrogen metabolism in Mycobacterium tuberculosis physiology and virulence. Nat Rev Microbiol 12, 729-737
Gouzy, A., Larrouy-Maumus, G., Bottai, D., Levillain, F., Dumas, A., Wallach, J.B., Caire-Brandli, I., de Chastellier, C., Wu, T.D., Poincloux, R., Brosch, R., Guerquin-Kern, J.L., Schnappinger, D., Sorio de Carvalho, L.P., Poquet, Y., Neyrolles, O. (2014) Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathog 10, e1003928 (View)
Gouzy, A., Larrouy-Maumus, G., Wu, T.D., Peixoto, A., Levillain, F., Lugo-Villarino, G., Guerquin-Kern, J.L., de Carvalho, L.P., Poquet, Y., Neyrolles, O. (2013) Mycobacterium tuberculosis nitrogen assimilation and host colonization require aspartate. Nat Chem Biol 9, 674-676 (View)
Botella, H., Stadthagen, G., Lugo-Villarino, G., de Chastellier, C., Neyrolles, O. (2012) Metallobiology of host-pathogen interactions: an intoxicating new insight. Trends Microbiol 20, 106-112
Botella, H., Peyron, P., Levillain, F., Poincloux, R., Poquet, Y., Brandli, I., Wang, C., Tailleux, L., Tilleul, S., Charriere, G.M., Waddell, S.J., Foti, M., Lugo-Villarino, G., Gao, Q., Maridonneau-Parini, I., Butcher, P.D., Castagnoli, P.R., Gicquel, B., de Chastellier, C., Neyrolles, O. (2011) Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10, 248-259 (View)
Brodin, P., Poquet, Y.*, Levillain, F.*, Peguillet, I.*, Larrouy-Maumus, G., Gilleron, M., Ewann, F., Christophe, T., Fenistein, D., Jang, J., Jang, M.S., Park, S.J., Rauzier, J., Carralot, J.P., Shrimpton, R., Genovesio, A., Gonzalo-Asensio, J.A., Puzo, G., Martin, C., Brosch, R., Stewart, G.R., Gicquel, B., Neyrolles, O. (2010) High content phenotypic cell-based visual screen identifies Mycobacterium tuberculosis acyltrehalose-containing glycolipids involved in phagosome remodeling. PLoS Pathog 6, e1001100 (View)
Becq, J., Gutierrez, M.C., Rosas-Magallanes, V., Rauzier, J., Gicquel, B., Neyrolles, O.** Deschavanne, P.** (2007) Contribution of horizontally acquired genomic islands to the evolution of the tubercle bacilli. Mol Biol Evol 24, 1861-1871 (View)
Rosas-Magallanes, V., Deschavanne, P., Quintana-Murci, L., Brosch, R., Gicquel, B., Neyrolles, O. (2006) Horizontal transfer of a virulence operon to the ancestor of Mycobacterium tuberculosis. Mol Biol Evol 23, 1129-1135 (View)
A nice paper you may want to read…
Neyrolles, O., Hernandez-Pando, R., Pietri-Rouxel, F., Fornes, P., Tailleux, L., Barrios Payan, J.A., Pivert, E., Bordat, Y., Aguilar, D., Prevost, M.C., Petit, C., Gicquel, B. (2006) Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One 1, e43 (View)
We are extremely proud of our former research assistants, PhD students and post-doctoral fellows who are now continuing their career in the best labs, enterprises and institutions in life sciences!
- Tamara Sneperger | Post-doctoral fellow, University of Edinburgh, UK
- Aurélien Boyancé | Project manager, Evotec, Toulouse, France
- Benjamin Raymond | Post-doctoral fellow, University of Newcastle, UK
- Yves-Marie Boudhen | Post-doctoral fellow, IRIM, Montpellier, France
- Alison Charton | Research assistant, Evotec, Toulouse, France
- Dan Corral | Post-doctoral fellow, NIAID, NIH, Bethesda, MD, USA
- Giulia Trimaglio | Post-doctoral fellow, Institute for Clinical Chemistry and Laboratory Medicine, Faculty of Medicine, Technische Universität Dresden, Germany
- Lucie Bernard-Raichon | Post-doctoral fellow, New York University, NY, USA
- Maeva Dupont | Post-doctoral fellow, University of Oxford, UK
- Alexia Dumas | Post-doctoral fellow, University College Dublin, Ireland – Present: Post-doctoral fellow, IRSD, Toulouse, France
- Karine Pingris | Research engineer, Evotec, Toulouse, France
- André Colom | Research assistant, Evotec, Toulouse, France
- Danilo Pietretti | Project officer, European Commission
- Alan Bénard | Post-doctoral fellow, University Hospital, Erlangen, Germany
- Anthony Troegeler | Research associate, Evotec, Toulouse, France
- Anna Grabowska | Associate professor, Medical University of Warsaw, Warsaw, Poland
- Kanjana Nukdee | Professor, Ubon Ratchathani University, Thailand
- Ingrid Mercier | Business Project Leader, Airbus group, Toulouse, France
- Alexandre Gouzy | Post-doctoral fellow, Weill Cornell Medical College, NY, USA
- Hélène Botella | Post-doctoral fellow, Weill Cornell Medical College, NY, USA – Present: Post-doctoral fellow, Imperial College, London, UK
- Antoine Tanne | Immune Modulatory Drug Development Specialist (I/O – Vaccines), Boston, MA
- Jichan Jang | Professor, Gyeongsang National University, Jinju, South Korea
- Chongzhen Wang | Associate professor, Guilin Medical College, Guilin, China
- Chuan Wang | Fudan University, Shanghai, China

