Molecular Mycobacterial Pathogenesis

Group Leader
The overall objectives of our group are to understand what make some mycobacterial species, such as Mycobacterium tuberculosis and Mycobacterium abscessus, such deadly human pathogens and to use this knowledge to propose new ways to fight them. The biosynthesis and translocation to the cell surface of pathogen specific lipids and their contribution to pathogenesis, and the emergence and evolution of mycobacterial pathogens have been the main focus of this group in the recent years.
Our team develops innovative genetic approaches to understand the exceptional epidemic success of tuberculosis bacilli and related mycobacterial pathogens.

One of the key features contributing to the success of Mycobacterium tuberculosis as a human pathogen is its highly complex cell envelope and the unique structure of some of its constituents. A major focus of our research has been determining how mycobacterial lipids are assembled and translocated to the bacterial cell surface, and how those specific to pathogenic mycobacteria subvert the host immune response to benefit the pathogen. We recently described the biosynthetic pathway for the largest known mycobacterial glycolipids and established its close relationship with that of M. tuberculosis specific lipids. We characterized novel molecular mechanisms of action of mycobacterial lipid virulence factors and found that they modulate the innate immune response either through direct binding to macrophage receptors or through insertion within host membrane.
A second important area of our research is related to evolution of M. tuberculosis. This microorganism evolved from an environmental ancestor and clonally expanded in the human population. We explore the emergence of this pathogen and the specific adaptations favoring persistence and aerosol transmission in humans, two major features to explain the epidemic success of M. tuberculosis. We demonstrated that evolution toward a strict pathogenic lifestyle was associated with remodeling of the cell envelope leading to modification of surface lipid composition and to a reduced permeability to toxic molecules.
Finally, although our focus is mostly on TB bacilli, we also expand our studies to another mycobacterial emerging pathogen, Mycobacterium abscessus, to understand the mechanisms explaining its exceptional antibiotic tolerance and its deleterious capacity to colonize the lungs of cystic fibrosis patient.
Our scientific strategy relies on innovative genetic approaches and complementary expertise from the team members in molecular genetics, microbiology, biochemistry, cellular microbiology, and animal experimentation.
Team members
Research Scientists
Christophe Guilhot (CNRS)
Hélène Botella (University)
Kaymeuang Cam (University)
Christian Chalut (CNRS)
Research Engineers
Marie Devaere
Sophie Manzi (CNRS)
Jérémy Sintes
Postdoctoral Fellows
Henrich Gasparovic
Carlos Adriano de Matos e Silva
Valentin Wasselin
PhD Students
Grégoire Mongin
Allen et al. (2021) Parallel experimental evolution in mice reveals that increased resistance was a key event for the emergence of persistent tuberculosis bacilli. Nat Microbiol
Augenstreich et al. (2019) The conical shape of DIM lipids promotes Mycobacterium tuberculosis infection of macrophages. Proc Natl Acad Sci USA
Burbaud et al. (2016) Trehalose polyphleate, are produced by a glycolipid biosynthetic pathway conserved across phylogenetically distant mycobacteria. Cell Chem Biol
Boritsch et al.(2016) pks5-recombination-mediated cell surface remodelling in Mycobacterium tuberculosis emergence. Nat Microbiol
Gonzalo-Asensio et al. (2014) Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc Natl Acad Sci USA
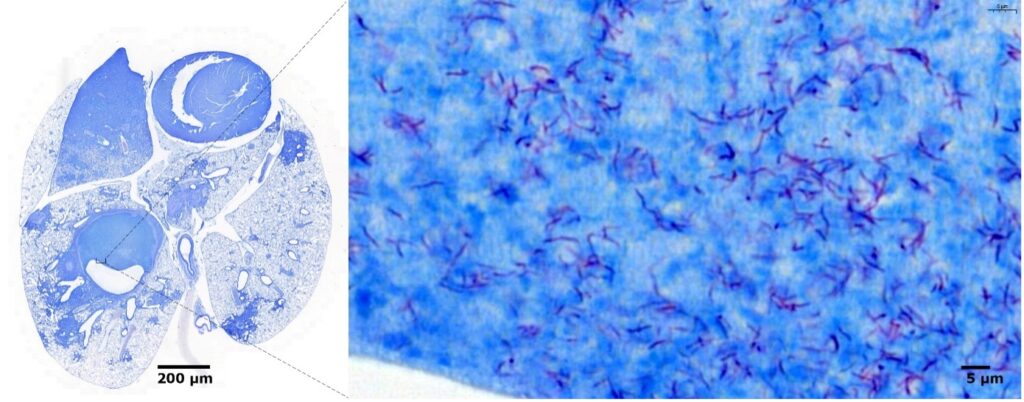
Lung necrotic lesions generated in the murine model by the tubercle bacillus. The sections were stained by the Ziehl-Neelsen method and reveal the bacteria in pink that persist in the core of the necrotic area of the lesion (in blue).
Collaborations
- Odile Burlet-Schiltz, Matthieu Chavent, Etienne Meunier, Lionel Mourey, Olivier Neyrolles, Jérôme Nigou, & Antonio Peixoto Institut de Pharmacologie et de Biologie Structurale, Toulouse, France
- Michel Baltas Laboratoire de Chimie de Coordination, Toulouse, France
- Caroline Demangel & Roland Brosch Institut Pasteur, Paris, France
- Alain Baulard & Philip Supply Centre d’Infection et d’Immunité de Lille, France
- Laurent Kremer Institut de Recherche en Infectiologie de Montpellier, France
- Carlos Martin & Jesus Gonzalo-Asensio, University of Zaragza, Spain
- Gerald Larrouy-Maumus Imperial College, London, UK
- Leila Mendonça-Lima Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
- Daria Bottai University of Pisa, Pisa, Italy
- Sung Jae Shin Yongsei University, Seoul, Korea
- Andreas Kupz James Cook University, Cairns, Australia
Funding
The team is supported by several grants from:
- MSDAVENIR
- Agence Nationale de la Recherche
- Fondation Bettencourt Schueller
- Vaincre la Mucoviscidose
- Bill & Melinda Gates Foundation
Our team is officially labelled by the Fondation pour la Recherche Médicale (2022-2025).
This is short list of our main recent publications. All publications and reviews are available through Pubmed.
2023
Wetzel KS, Illouz M, Abad L, Aull HG, Russell DA, Garlena RA, Cristinziano M, Malmsheimer S, Chalut C, Hatfull GF and L Kremer (2023). Therapeutically useful mycobacteriophages BPs and Muddy require trehalose polyphleates. Nat Microbiol 8:1717-1731
Leon-Icaza SA, Bagayoko S, Vergé R, Iakobachvili N, Ferrand C, Aydogan T, Bernard C, Sanchez Dafun A, Murris-Espin M, Mazières J, Bordignon, PJ, Mazères S, Bernes-Lasserre P, Ramé V, Lagarde JM, Marcoux J, Bousquet MP, Chalut C, Guilhot C, Clevers H, Peters PJ, molle V, Lugo-Villarino G, Cam K, Berry L, Meunier E and C Cougoule (2023) Druggable redox pathways against Mycobacterium abscessus in cystic fibrosis patient-derived airway organoids. PLOS Pathog 19:e1011559
Malaga W, Payros D, Meunier E, Frigui W, Sayes F, Pawlik A, Orgeur M, Berrone C, Moreau F, Mazères S, Gonzalo-Asensio J, Rengel D, Martin C, Astarie-Dequeker C, Mourey L, Brosch R and C Guilhot (2023) Natural mutations in the sensor kinase of the PhoPR two-component regulatory system modulate virulence of ancestor-like tuberculosis bacilli. PLOS Pathog 19:e1011437
Thouvenel L, Rech J, Guilhot C, Bouet JY and C Chalut (2023) In vivo imaging of MmpL transporters reveals distinct subcellular locations for export of mycolic acids and non-essential trehalose polyphleates in the mycobacterial outer membrane. Sci Rep 13:7045
2022
Bon, C., Cabantous, S., Julien, S., Guillet, V., Chalut, C., Rima, J., Brison, Y., Malaga, W., Sanchez-Dafun, A., Gavalda, S., Quemard, A., Marcoux, J., Waldo, G.S., Guilhot, C., Mourey, L. (2022) Solution structure of the type I polyketide synthase Pks13 from Mycobacterium tuberculosis. BMC Biol 20, 147
Iakobachvili, N., Leon-Icaza, S.A., Knoops, K., Sachs, N., Mazeres, S., Simeone, R., Peixoto, A., Bernard, C., Murris-Espin, M., Mazieres, J., Cam, K., Chalut, C., Guilhot, C., Lopez-Iglesias, C., Ravelli, R.B.G., Neyrolles, O., Meunier, E., Lugo-Villarino, G., Clevers, H., Cougoule, C., Peters, P.J. (2022) Mycobacteria-host interactions in human bronchiolar airway organoids. Mol Microbiol 117, 682-692
2021
Payros, D., Alonso, H., Malaga, W., Volle, A., Mazeres, S., Dejean, S., Valiere, S., Moreau, F., Balor, S., Stella, A., Combes-Soia, L., Burlet-Schiltz, O., Bouchez, O., Nigou, J., Astarie-Dequeker, C., Guilhot, C. (2021) Rv0180c contributes to Mycobacterium tuberculosis cell shape and to infectivity in mice and macrophages. PLoS Pathog 17, e1010020
Carivenc, C., Maveyraud, L., Blanger, C., Ballereau, S., Roy-Camille, C., Nguyen, M.C., Genisson, Y., Guilhot, C., Chalut, C., Pedelacq, J.D., Mourey, L. (2021) Phosphopantetheinyl transferase binding and inhibition by amidino-urea and hydroxypyrimidinethione compounds. Sci Rep 11, 18042
Allen, A.C., Malaga, W., Gaudin, C., Volle, A., Moreau, F., Hassan, A., Astarie-Dequeker, C., Peixoto, A., Antoine, R., Pawlik, A., Frigui, W., Berrone, C., Brosch, R., Supply, P., Guilhot, C. (2021) Parallel in vivo experimental evolution reveals that increased stress resistance was key for the emergence of persistent tuberculosis bacilli. Nat Microbiol 6, 1082-1093
Schwarz, M.G.A., Antunes, D., Correa, P.R., da Silva-Goncalves, A.J., Malaga, W., Caffarena, E.R., Guilhot, C., Mendonca-Lima, L. (2020) Mycobacterium tuberculosis and M. bovis BCG Moreau Fumarate Reductase Operons Produce Different Polypeptides That May Be Related to Non-canonical Functions. Front Microbiol11, 624121
2020
Grabowska, A.D., Brison, Y., Maveyraud, L., Gavalda, S., Faille, A., Nahoum, V., Bon, C., Guilhot, C., Pedelacq, J.D., Chalut, C., Mourey, L. (2020) Molecular Basis for Extender Unit Specificity of Mycobacterial Polyketide Synthases. ACS Chem Biol 15, 3206-3216
Augenstreich, J., Haanappel, E., Sayes, F., Simeone, R., Guillet, V., Mazeres, S., Chalut, C., Mourey, L., Brosch, R., Guilhot, C., Astarie-Dequeker, C. (2020) Phthiocerol Dimycocerosates From Mycobacterium tuberculosis Increase the Membrane Activity of Bacterial Effectors and Host Receptors. Front Cell Infect Microbiol 10, 420
Schwarz, M.G.A., Correa, P.R., Malaga, W., Guilhot, C., Mendonca-Lima, L. (2020) Mycobacterium bovis BCG moreau is naturally deficient in homologous recombination. Tuberculosis (Edinb) 123, 101956
Thouvenel, L., Prevot, G., Chiaradia, L., Parra, J., Mouton-Barbosa, E., Locard-Paulet, M., Marcoux, J., Tropis, M., Burlet-Schiltz, O., Daffe, M., Guilhot, C., Etienne, G., Chalut, C. (2020) The final assembly of trehalose polyphleates takes place within the outer layer of the mycobacterial cell envelope. J Biol Chem 295, 11184-11194
Alonso, M.N., Malaga, W., Mc Neil, M., Jackson, M., Romano, M.I., Guilhot, C., Santangelo, M.P. (2020) Efficient method for targeted gene disruption by homologous recombination in Mycobacterium avium subspecie paratuberculosis. Res Microbiol 171, 203-210
Bah, A., Sanicas, M., Nigou, J., Guilhot, C., Astarie-Dequeker, C., Vergne, I. (2020) The Lipid Virulence Factors of Mycobacterium tuberculosis Exert Multilayered Control over Autophagy-Related Pathways in Infected Human Macrophages. Cells 9, 666
Levillain, F., Kim, H., Woong Kwon, K., Clark, S., Cia, F., Malaga, W., Lanni, F., Brodin, P., Gicquel, B., Guilhot, C., Bancroft, G.J., Williams, A., Jae Shin, S., Poquet, Y., Neyrolles, O. (2020) Preclinical assessment of a new live attenuated Mycobacterium tuberculosis Beijing-based vaccine for tuberculosis. Vaccine 38, 1416-1423
Nguyen, M.C., Saurel, O., Carivenc, C., Gavalda, S., Saitta, S., Tran, M.P., Milon, A., Chalut, C., Guilhot, C., Mourey, L., Pedelacq, J.D. (2020) Conformational flexibility of coenzyme A and its impact on the post-translational modification of acyl carrier proteins by 4′-phosphopantetheinyl transferases. FEBS J 287, 4729-4746
2019
Doz-Deblauwe, E., Carreras, F., Arbues, A., Remot, A., Epardaud, M., Malaga, W., Mayau, V., Prandi, J., Astarie-Dequeker, C., Guilhot, C., Demangel, C., Winter, N. (2019) CR3 Engaged by PGL-I Triggers Syk-Calcineurin-NFATc to Rewire the Innate Immune Response in Leprosy. Front Immunol 10, 2913
Augenstreich, J., Haanappel, E., Ferre, G., Czaplicki, G., Jolibois, F., Destainville, N., Guilhot, C., Milon, A., Astarie-Dequeker, C.*, Chavent, M.* (2019) The conical shape of DIM lipids promotes Mycobacterium tuberculosis infection of macrophages. Proc Natl Acad Sci USA 116, 25649-25658
2018
Diaz Acosta, C.C., Dias, A.A., Rosa, T., Batista-Silva, L.R., Rosa, P.S., Toledo-Pinto, T.G., Costa, F., Lara, F.A., Rodrigues, L.S., Mattos, K.A., Sarno, E.N., Bozza, P.T., Guilhot, C., de Berredo-Pinho, M., Pessolani, M.C.V. (2018) PGL I expression in live bacteria allows activation of a CD206/PPARgamma cross-talk that may contribute to successful Mycobacterium leprae colonization of peripheral nerves. PLoS Pathog 14, e1007151
Oldenburg, R., Mayau, V., Prandi, J., Arbues, A., Astarie-Dequeker, C., Guilhot, C., Werts, C., Winter, N., Demangel, C. (2018) Mycobacterial Phenolic Glycolipids Selectively Disable TRIF-Dependent TLR4 Signaling in Macrophages. Front Immunol 9, 2
2017
Kaufmann, S.H.E., et al. (2017) TBVAC2020: Advancing tuberculosis vaccines from discovery to clinical development. Front Immunol 8, 1203
Alonso, H., Parra, J., Malaga, W., Payros, D., Liu, C.F., Berrone, C., Robert, C., Meunier, E., Burlet-Schiltz, O., Riviere, M., Guilhot, C. (2017) Protein O-mannosylation deficiency increases LprG-associated lipoarabinomannan release by Mycobacterium tuberculosis and enhances the TLR2-associated inflammatory response. Sci Rep 7, 7913
Augenstreich, J., Arbues, A., Simeone, R., Haanappel, E., Wegener, A., Sayes, F., Le Chevalier, F., Chalut, C., Malaga, W., Guilhot, C., Brosch, R.*, Astarie-Dequeker, C.* (2017) ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol 19, 12726
2016
Arbues, A., Malaga, W., Constant, P., Guilhot, C., Prandi, J., Astarie-Dequeker, C. (2016) Trisaccharides of Phenolic Glycolipids Confer Advantages to Pathogenic Mycobacteria through Manipulation of Host-Cell Pattern-Recognition Receptors. ACS Chem Biol 11, 2865-2875
Burbaud, S., Laval, F., Lemassu, A., Daffe, M., Guilhot, C., Chalut, C. (2016) Trehalose Polyphleates Are Produced by a Glycolipid Biosynthetic Pathway Conserved across Phylogenetically Distant Mycobacteria. Cell Chem Biol 23, 278-289
Boritsch, E.C., Frigui, W., Cascioferro, A., Malaga, W., Etienne, G., Laval, F., Pawlik, A., Le Chevalier, F., Orgeur, M., Ma, L., Bouchier, C., Stinear, T.P., Supply, P., Majlessi, L., Daffe, M., Guilhot, C.*, Brosch, R.* (2016) pks5-recombination-mediated surface remodelling in Mycobacterium tuberculosis emergence. Nat Microbiol1, 15019
Post-Docs
Aideen Allen, Ireland (2017-2020)
Delphine Payros, France (2017-2019)
Henar Alonso, Spain (2013-2016)
Ainhoa Arbues, Spain (2012-2015)
Anna Grabowska, Poland (2011-2012)
Sabine Gavalda, France (2009-2010)
Guilaume Tabouret, France (2007-2008)
Esther Perez, Spain (2002-2004)
PhDs
Laurie Thouvenel, France (2017-2020)
Marcos Schwarz, Brazil, visiting PhD student (2018-2019)
Jacques Augenstreich, France (2014-2018)
Sophie Burbaud, France (2011-2015)
Charlotte Passemar, France (2009-2013)
Cécile Leblanc, France (2008-2012)
Roxane Siméone, France (2004-2008)
Gaëlle Huet, France (2005-2009)
Damien Portevin, France (2001-2005)
Research assistants
Coralie Roy-Camille, France (2019-2020)
Alice Marchand, France (2017-2019)
Gautier Prévot, France (2016-2018)
Céline Berrone, France (2015-2018)
Arnaud Volle, France (2015-2017)
Stéphane Saitta, France (2014-2015)
Flavie Moreau, France (2010-2014)
Thomas Prudhomme, France (2009-2011)

