Endothelial cells in Immunity, Inflammation and Cancer

Group Leader
Our team is among the world leaders for the characterization of high endothelial venules (HEVs), portals of entry for lymphocytes into lymphoid organs, inflamed and tumor tissues, and for the study of interleukin-33 (IL-33), a nuclear alarmin from the IL-1 cytokine family that we discovered in 2003, which plays crucial roles in innate immunity and allergic inflammation.
The very unique, long-standing and multidisciplinary expertise of our team is a key asset to study the roles of HEVs and IL-33 in immunity, inflammation and cancer.

We demonstrated that dendritic cells, which are well known for their role as antigen-presenting cells, play an unexpected and important role in the regulation of HEV differentiation and function in lymph nodes (Nature 2011). Using single-cell RNA sequencing, we revealed the cellular and spatial heterogeneity of HEVs in lymph nodes, and we identified the genes modulated after inhibition of the dialog between HEVs and dendritic cells (Cell Reports 2019). A decade ago, we discovered the frequent presence of HEV-like blood vessels in human solid tumors (designated Tumor-Associated HEVs, TA-HEVs), and their association with cytotoxic lymphocyte infiltration and favourable clinical parameters (Cancer Res 2011, 311 citations). Our study introduced a novel concept in cancer biology “Blood vessels in human tumors are not all the same and specific subsets (i.e., TA-HEVs) can contribute to tumor suppression rather than tumor growth”. Using intravital microscopy, we recently showed that TA-HEVs are major sites of lymphocyte entry into tumors during anti-tumor immune responses and cancer immunotherapy, exhibit a unique bi-functional hybrid phenotype, and are associated with better clinical response and survival of metastatic melanoma patients treated with anti-PD-1/anti-CTLA-4 cancer immunotherapy (Cancer Cell 2022). We believe that novel therapeutic strategies based on the modulation of TA-HEVs could have a major impact on antitumor immunity, response to cancer immunotherapy, and clinical outcome of cancer patients.
IL-33 is a chromatin-associated cytokine from the IL-1 family that we initially discovered as a major nuclear factor of HEV (NF-HEV) (PNAS 2007, 765 citations). We showed that IL-33 is a tissue-derived nuclear cytokine constitutively expressed to high levels in blood vessels and epithelial barrier tissues (PLoS 2008, 875 citations). We demonstrated that IL-33 functions as an alarm signal (alarmin) released upon cell damage to alert immune cells expressing the IL-33 receptor ST2 (PNAS 2009, 510 citations). These include various cell types involved in type-2 immunity and allergic inflammation such as mast cells, and group 2 innate lymphoid cells (ILC2s). We demonstrated that inflammatory proteases can generate truncated forms of IL-33 that are 30-fold more potent than the full length protein for activation of group 2 innate lymphoid cells (PNAS 2012, 389 citations; PNAS 2014). Recently, we discovered that full length IL-33 functions as a protease sensor that detects proteolytic activities associated with a large variety of environmental allergens (Nat Immunol 2018, 174 citations). An important objective of our team is to further characterize IL-33 regulation and mechanisms of action in vivo, through the use of multidisciplinary approaches.
Team members
Research Scientists
Corinne Cayrol (CNRS)
Jean-Philippe Girard (Inserm)
Emma Lefrançais (CNRS)
Nathalie Ortéga (University)
Research Engineers
Elisabeth Bellard (CNRS)
Pascale Mercier (CNRS)
Stéphane Roga (CNRS)
Clara Saint-Martin
Dorian Tarroux
Postdoctoral Fellow
Jade Hebras
PhD Students
Léa Fromont
Lucie Gelon
Jerko Ljubetic
Estefania Vina-Barrientos
Our research projects
Asrir*, Tardiveau*, Coudert*, Laffont*, Blanchard* et al. (2022) Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy. Cancer Cell
Cayrol*, Duval* et al. (2018) Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol
Lefrançais*, Duval* et al. (2014) Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA
Martinet*, Garido* et al. (2011) Human solid tumors contain high endothelial venules (HEV): association with T and B lymphocyte infiltration and favourable prognosis in breast cancer. Cancer Res
Moussion and Girard (2011) Dendritic cells control lymphocyte entry to lymph nodes via high endothelial venules. Nature
Cayrol and Girard (2009) The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA
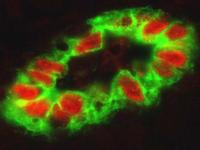
High endothelial cells from HEVs (green) are characterized by a plump morphology and express high levels of the alarmin cytokine IL-33 (red). © Jean-Philippe Girard

Jean-Philippe Girard ranks among the world’s most cited scientists in the prestigious ‘Highly Cited Researchers’ list in 2019, 2020, 2022 and 2023 in the Immunology category.
Jean-Philippe Girard is among the Highly Cited Researchers 2022 in the Immunology category
Specialist in the analysis of scientific production, Clarivate identifies each year the most influential researchers in the world. The “Highly Cited Researchers” are thus the researchers whose work is the most cited, typically those having several articles in the top 1% of citations in their field during the last decade in the “Web of science ™”.
This list is used in the famous ranking of universities in Shanghai.
Once again a member of the list of “Highly Cited Researchers” (2022), Jean-Philippe Girard, Inserm research director, head of the team “Vascular Biology: Endothelial cells in Immunity, Inflammation and Cancer” at the IPBS-Toulouse (CNRS-Université Paul Sabatier), is, for the third time, one of the most influential researchers in Immunology.

Corinne Cayrol, laureate of the National Academy of Medicine
On December 17th in Paris, Dr. Corinne Cayrol received the Edouard Bonnefous Prize 2019 from the National Academy of Medicine for her work on Interleukin-33 (IL-33) as an important therapeutic target for asthma, regulated by environmental allergens.
This prize rewards all work on the environment and its consequences on human health.

Jean-Philippe Girard, winner of the Prize Jean Valade 2018 from Fondation de France
Jean-Philippe GIRARD, Silver Medal of the CNRS 2013, Inserm Research Director at IPBS (CNRS-University of Toulouse), will receive the Prize Jean Valade 2018 from ‘Fondation de France’ on March 28th at the Collège de France in Paris, for his work on IL-33, a major therapeutic target for asthma and allergic diseases.
Jean-Philippe Girard is honored for his pioneering work on the discovery and characterization of IL-33, a protein that is now recognized as a central mediator of allergic inflammation and asthma. Asthma is one of the most common chronic inflammatory diseases in humans, affecting up to 300 million people worldwide. Progress in the prevention and treatment of asthma and allergic diseases is thus urgently needed. Identification and validation of novel therapeutic targets is critical in order to develop future treatments for those who do not respond to available therapies. The work of Jean-Philippe Girard and his team over the past 15 years has significantly contributed to the current knowledge about IL-33 mechanisms of action and regulation and the validation of IL-33 as a bona fide therapeutic target for asthma and other allergic diseases, such as atopic dermatitis.


Emma Lefrançais receives the “Grand Prix Jeune Chercheur” from the FONROGA Foundation (Dec. 2018)
Emma Lefrançais, CNRS Research associate in the laboratory of Jean-Philippe Girard, received one of the two “Grand Prix Jeune Chercheur” from the FONROGA Foundation.
Using intravital lung imaging, Emma’s work focuses on the role of IL-33 in inflammation and cellular dynamics during respiratory allergies.
The FONROGA Foundation supports initiatives and achievements in the fields of culture and health in the Toulouse region. Since its creation in 2016, more than €160,000 have been given to young researchers through Awards and fellowships. At IPBS, the Foundation has already given the “Grand Prix Jeune Chercheur” to three young researchers (Drs. Laure Gibot, Etienne Meunier and Emma Lefrançais).

Jean-Philippe Girard, recipient of the Jean-Paul Binet Prize from “Fondation pour la Recherche Médicale”
Dr. Jean-Philippe GIRARD, received the Jean-Paul Binet Prize 2016 on December 5th, at the “Fondation pour la Recherche Médicale” in Paris.
This Prize rewards researchers who have made important advances in the understanding of the cardiovascular system. Jean-Philippe Girard received the prize for his work on HEV blood vessels and their role in inflammation and cancer.

Jean-Philippe Girard, Director of IPBS, winner of the prestigious Prize in Cancerology “Gallet et Breton” from the National Academy of Medecine
Dr. Jean-Philippe GIRARD received the prize “Gallet et Breton” on December 17th 2013, at the National Academy of Medecine in Paris. This prestigious Prize in Cancerology rewards researchers who have made important advances in cancer research or cancer therapy. Jean-Philippe Girard received the prize for his work on HEV blood vessels and their role in cancer.
Jean-Philippe Girard, Silver Medal of the CNRS
The CNRS Silver Medal honors researchers who are only at the beginning of their rise to fame, but who are already recognized nationally and internationally for the originality, quality, and importance of their work.
Jean-Philippe Girard, Director of IPBS, received this prestigious award for his work on the role of blood vessels in inflammation and cancer.


Jean-Philippe Girard, winner of the prestigious prize “Grand Prix René Turpin de Cancérologie” from the National Academy of Sciences
On October 16th 2012, Dr. Jean-Philippe Girard received the prestigious Prize in Cancerology, delivered every two years by the National Academy of Sciences and the ‘Institut de France’, for his work on HEV blood vessels and their role in cancer.
Jean-Philippe Girard received the first “Stars of the Health” (Étoiles de la Santé) Cancer Research Prize 2012
On 27 September 2012, Dr. Jean-Philippe GIRARD, Director of IPBS, received the first “Stars of the Health (Étoiles de la Santé) Cancer Research Prize” at the ’Cité de l’Espace’ in Toulouse, France.
The “Stars of the Health Prizes” were delivered for the first time this year by Foundation Oréade-Prévifrance. This event aimed at celebrating clinical, therapeutic and scientific research actors as well as start-up, pharmaceutical companies and associations working in the field of “Health and Medicine” in the south-west of France.
Five awards were delivered including “The Cancer Research Award” which rewards teams involved in fundamental or clinical research in the field of prevention and treatment of cancer. This award was given to Dr. Jean-Philippe Girard and his team for their work on HEV blood vessels in human cancer.
Dr. Girard receives the “”Stars of the Health Cancer Research Prize 2012″” at the ’Cité de l’Espace’ in Toulouse on September 27th (in French).


The “Ruban Rose Avenir 2011” Prize awarded to Jean-Philippe Girard
Dr. Jean-Philippe Girard, Director of IPBS and Head of the team “Vascular biology : endothelial cells, inflammation and cancer” is the recipient of the “Ruban Rose Avenir 2011 Prize”.
The“Ruban Rose Prizes” are awarded each year since 2004 to researchers who have made important contributions in breast cancer research.
- Fanny Lafouresse received in 2015 a travel grant and the best oral presentation award (Biolegend) at the World Immune Regulation meeting IX (Davos, Switzerland).
- Anaïs Duval received in 2014 the best poster prize from ’Nature Immunology’ at the EMBO conference in Innate Lymphoid Cells (Pasteur, Paris).
- Fanny Lafouresse received the best oral communication prize 2013 from the SILLC association.
- Christine Moussion received the Paul Sabatier Prize 2012 from the “Académie des Sciences, Inscriptions et Belles Lettres de Toulouse”.
- The European Cytokine Society Prize 2012 (Junior Publication Initiative) was awarded to Emma Lefrançais.
- Ludovic Martinet and Emma Lefrançais received the best poster and oral communication prize 2011 from the “Société Française d’Angiogenèse”.
- Emma Lefrançais received the best poster prize 2011 from the Cold Spring Harbor Asia Conference ’Infection and Immunity’.
The complete list of our publications is available through Pubmed
Main publications on the HEV topic
- Martinet, L.*, Garrido, I.*, Filleron, T., Le Guellec, S., Bellard, E., Fournie, J.J., Rochaix, P., Girard, J.P. (2011) Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 71, 5678-5687 (Press release from CNRS/INSERM; This article has been highlighted in Cancer Res 71:5601-5; 311 citations in Web of Science, WoS)
We went against the dogma that ‘blood vessels facilitate tumor growth’ and introduced a novel concept in cancer biology: ‘Blood vessels in human tumors are not all the same and some types of blood vessels (i. e. tumor-associated HEVs) may contribute to the fight against cancer’.
- Moussion, C., Girard, J.P. (2011) Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 479, 542-546 (Press release from CNRS/INSERM; 210 citations in WoS)
We showed that dendritic cells, which are well known for their role as antigen-presenting cells, play an unexpected and important role in the maintenance of HEV blood vessels in adult lymphoid tissues.
- Martinet, L., Le Guellec, S., Filleron, T., Lamant, L., Meyer, N., Rochaix, P., Garrido, I., Girard, J.P. (2012) High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 1, 829-839 (137 citations in WoS)
We found that high densities of tumor-associated HEVs are correlated with high levels of lymphocyte infiltration and favorable clinical characteristics in primary human melanomas.
- Lafouresse, F., Bellard, E., Laurent, C., Moussion, C., Fournie, J.J., Ysebaert, L., Girard, J.P. (2015) L-selectin controls trafficking of chronic lymphocytic leukemia cells in lymph node high endothelial venules in vivo. Blood126, 1336-1345 (Press release from CNRS/INSERM; This article has been selected by the Editors for the cover of the journal and a research highlight in “Inside Blood” 2015, 126:1267-1268)
We used intravital microscopy in mouse to study the in vivo trafficking in HEVs of human leukemic cells from chronic lymphocytic leukemia patients.
- Veerman, K., Tardiveau, C., Martins, F., Coudert, J., Girard, J.P. (2019) Single-Cell Analysis Reveals Heterogeneity of High Endothelial Venules and Different Regulation of Genes Controlling Lymphocyte Entry to Lymph Nodes. Cell Rep 26, 3116-3131 e3115
We reported the 1st characterization of the full-length HEV transcriptome by single cell RNA-sequencing, discovered the heterogeneity of HEV endothelial cells in homeostatic and inflamed lymph nodes and revealed important differences in the regulation of HEV genes after interruption of LTbR signaling.
- Asrir, A.#, Tardiveau, C.#, Coudert, J.#, Laffont, R.#, Blanchard, L.#, Bellard, E., Veerman, K., Bettini, S., Lafouresse, F., Vina, E., Tarroux, D., Roy, S., Girault, I., Molinaro, I., Martins, F., Scoazec, J.Y., Ortega, N., Robert, C., Girard, J.P. (2022) Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy. Cancer Cell 40, 318-334 e319 (#Co-first authors)
Using intravital microscopy, we demonstrated that TA-HEVs are major sites of lymphocyte entry into tumors during anti-tumor immune responses and cancer immunotherapy. We also showed that TA-HEVs are associated with better clinical response and survival of metastatic melanoma patients treated with anti-PD-1/anti-CTLA-4 dual immunotherapy.
Reviews on the HEV topic
- Girard, J.P.*, Moussion, C., Forster, R. (2012) HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 12, 762-773 (* Corresponding author; 435 citations in WoS, Top 1%)
We were invited by the Nature Rev Immunol Chief Editor to write a comprehensive review on the role of HEV blood vessels in lymphocyte migration to lymph nodes.
- Blanchard, L., Girard, J.P. (2021) High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 24, 719-753
We wrote a landmark review on the function and regulation of HEV blood vessels in immunity, inflammation and cancer (34 pages, 339 references).
Main publications on the IL-33 topic
- Baekkevold, E., Roussigné, M., Johansen, F.E., Jahnsen, F., Amalric, F., Erard, M., Brandtzaeg, P., Haraldsen, G., Girard, J.P. (2003) Molecular characterization of NF-HEV – a nuclear factor preferentially expressed in human high endothelial venules. Am J Path 163, 69-79 (354 citations in WoS)
We discovered a novel nuclear factor abundantly expressed in HEV endothelial cells in human lymphoid organs, that we designated nuclear factor of HEV (NF-HEV).
- Carriere, V.*, Roussel, L.*, Ortega, N., Lacorre, D.A., Americh, L., Aguilar, L., Bouche, G., Girard, J.P. (2007) IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA 104, 282-287 (*first co-autors; This article has been highlighted in Science Signalling 2007(390):pe31; 765 citations in WoS)
We showed that the IL-1-like cytokine IL-33 is a chromatin-associated cytokine, which is identical to NF-HEV, the nuclear factor that we cloned from purified HEV endothelial cells in 2003.
- Moussion, C.*, Ortega, N.*, Girard, J.P. (2008) The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 3, e3331 (*co-first authors, 875 citations in WoS)
We reported that IL-33 is constitutively expressed in blood vessels and epithelial barrier tissues, and we proposed the concept that IL-33 may function as a novel alarmin (alarm signal) rather than as a classical cytokine (we were the first to propose this concept).
- Roussel, L., Erard, M., Cayrol, C., Girard, J.P. (2008) Molecular mimicry between IL-33 and KSHV for attachment to chromatin through H2A-H2B acidic pocket. EMBO Rep 9, 1006-1012 (213 citations in WoS)
We discovered a unique example of molecular mimicry between a virus and a nuclear cytokine, and proposed that Kaposi’s sarcoma virus hijacked the chromatin-binding domain of IL-33 in order to maintain the viral genome in infected cells.
- Cayrol, C., Girard, J.P. (2009) The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA 106, 9021-9026 (This article has been highlighted in Immunity 2009, 31:5-7; 510 citations in WoS)
Contrary to the dogma in the field, we showed that IL-33 does not require maturation for biological activity and that cleavage by caspases results in IL-33 inactivation, rather than activation (We were the first to report these findings, which were later confirmed by 3 other teams).
- Lefrancais, E., Roga, S., Gautier, V., Gonzalez-de-Peredo, A., Monsarrat, B., Girard, J.P.*, Cayrol, C*. (2012) IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA 109, 1673-1678 (* Co-senior authors; Press release from CNRS/INSERM; 389 citations in WoS, Top 1%)
We showed that processing of IL-33 by inflammatory proteases generate mature forms of the cytokine with highly increased biological activity.
- Pichery, M.#, Mirey, E.#, Mercier, P., Lefrancais, E., Dujardin, A., Ortega, N.*, Girard, J.P.* (2012) Endogenous IL-33 Is Highly Expressed in Mouse Epithelial Barrier Tissues, Lymphoid Organs, Brain, Embryos, and Inflamed Tissues: In Situ Analysis Using a Novel Il-33-LacZ Gene Trap Reporter Strain. J Immunol 188, 3488-3495 (# Co-first authors; * Co-senior authors; 339 citations in WoS, Top 1%)
We reported the atlas of IL-33 protein expression and Il33 promoter activity in mouse embryos and adult tissues at steady state and during inflammation.
- Lefrancais, E.#, Duval, A.#, Mirey, E., Roga, S., Espinosa, E., Cayrol, C.*, Girard, J.P.* (2014) Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA 111, 15502-15507 (# co-first authors,* Co-senior authors; Press release from CNRS/INSERM; This article has been highlighted in JACI, 2015, 135:1-2; 246 citations in WoS).
We discovered that cleavage within the central domain of IL-33 generates truncated forms that are 30-fold more potent than the native protein for activation of ILC2s, the major targets of IL-33 in vivo.
- Cayrol, C.#, Duval, A.#, Schmitt, P., Roga, S., Camus, M., Stella, A., Burlet-Schiltz, O., Gonzalez-de-Peredo, A., Girard, J.P.* (2018) Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol 19, 375-385 (# Co-first authors; *Co-corresponding author; National press release from INSERM/CNRS; This article has been highlighted in Nature Immunol. 2018 News and Views 19:318-320 and Nature Rev Immunol 2018 May issue; Recommended in F1000Prime, 174 citations in WoS, Top 1%).
We discovered that IL-33 functions as an allergen protease sensor that detects proteolytic activities associated with various clinically relevant aeroallergens and initiates allergic inflammation shortly after allergen exposure.
Reviews on the IL-33 topic
- Cayrol, C., Girard, J.P. (2014) IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol 31, 31-37 (453 citations in WoS, Top 1%)
We were invited to write a state of the art review on IL-33 mode of action, regulation and functions in health and disease.
- Liew, F.Y., Girard, J.P., Turnquist, H.R. (2016) Interleukin-33 in health and disease. Nat Rev Immunol 16, 676-689 (617 citations in WoS, Top 1%)
We were invited to write a state of the art review on IL-33 protein and its roles in immunity and allergic inflammation.
- Cayrol, C., Girard, J.P. (2018) Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev 281, 154-168 (404 citations in WoS, Top 1%)
We were invited to write a state of the art review on IL-33 mode of action and regulation.
- Cayrol, C., Girard, J.P. (2022) Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 156, 155891
We were invited to make a critical review of IL-33 biology. Importantly, we alerted the scientific community to the problems of specificity of IL-33 reagents, and we made recommendations to generate reliable results.
Patent
Title : NOVEL SUPERACTIVE IL-33 FRAGMENTS, AND USES THEREOF
Publication N° WO/2012/113927
Publication Date: 30.08.2012
International Application No PCT/EP2012/053199
Applicants: CNRS
Inventors: GIRARD, Jean-Philippe;CAYROL-GIRARD, Corinne; LEFRANCAIS, Emma